The utility director at McCook, NE, reports the end of a 15-year-old nitrate contamination problem that increased in scope over the years to also include uranium and arsenic, through the installation of a multi-contaminant-removal, ion exchange treatment plant for the city’s drinking water. The new plant, serving a 6.8 mgd facility, started up in early 2006 and provided simultaneous removal of all three contaminants. A 1998 Administrative Order from the Nebraska Department of Health & Human Services was lifted eight months after the treatment plant startup.
“The long term upheaval is hopefully all behind us now,” said Jesse Dutcher, McCook utility director. “When a local regulator tells you that something needs to be fixed, you don’t want to wait until it becomes a federal problem. We had the U.S. Attorney General and U.S. EPA involved here, in addition to the Attorney General for Nebraska and the Nebraska Department of Environmental Quality, and it was very hard on the community politically as it struggled to find a solution to the contamination issue.”
The ion exchange treatment option was selected by a joint venture of W Design of McCook and Jacobson Helgoth Consultants of Denver, CO. Installation was handled by The Tonka Equipment Co. of Plymouth, MN, using ion exchange (IX) resins from The Purolite Co. of Bala Cynwyd, PA.
Jacobson Helgoth Consultants is now known as Jacobson Satchell Consultants.
“We initially considered six technology alternatives, finalized on reverse osmosis (RO) and IX, and then favored IX,” said Thomas T. Satchell, P.E., the primary engineer for the project. “But we had doubts about the ability of an IX media to remove all three contaminants, so we set up a pilot study. We used Purolite’s media in rented equipment, to prove their technology, and also to determine correct sizing for expected loading rates, and the amount of salt that would be needed for regeneration. After the pilot study proved a single media could remove all three contaminants, we put the IX option out for bid.”
The successful design and installation, completed within an exceptionally short mandated time frame, not only fulfilled the Consent Decree’s limitation of fines to $225,000 vs. a total accumulated liability of over $45 million, but also eliminated the assessment of further fines under the Decree of $65,000 per week. The fine liability had accumulated primarily during a long-term attempt to solve the problem through development of new water sources --- an attempt that was not formally abandoned until 2005.
“This was a pretty big feat for a community of only 8,000 people,” Dutcher said, “and to get it done in the short time frame was a tribute to the ability of the engineering company and the vendors to effectively coordinate their efforts.”
During the design stage, Purolite made extensive use of its proprietary IX Simulator software to model and predict the behavior of the contaminants, allowing the consultants to bypass several months of extensive piloting that would otherwise have been needed.
Dutcher, who took over as utility director for McCook in 2004, had been aware of the city’s contamination problem for many years during his tenure in municipal and state facility water management nearby. He sees the McCook IX treatment plant as representing a new frontier for ion exchange.
“Ion exchange technology has been widely known for a long time as a reliable method for water softening, which we also use it for, but it is less well known for contaminant removal,” he said. “Here, it not only served that function, but also expanded its reputation as strictly contaminant-specific, to also include handling more than one contaminant within the same process.
“We were in compliance for all three contaminant parameters within a couple of weeks after startup in February 2006, and it was just a matter of assuring it would also work well during the peak summer demand. The order was formally lifted in October 2006.”
The city’s 6.8 mgd drinking water production system is supplied by nine wells with a combined pumping capacity of 5500 gal./min., derived from individual well input ranging from 177 gal./min. to 1400 gal./min.
The system has a storage capacity of 6 million gallons. Average daily demand is 2.38 mgd, and historic peak is 9.572 mgd. Typical demand varies from 1.2 mgd during the winter to 5.2 mgd during the summer. Maximum system capacity is 13.92 mgd, and static pressure varies from 55-65 lbs/sq.in.
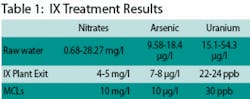
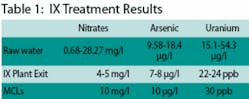
Raw water analyses at the wells show ranges of 0.68-28.27 mg/l for nitrates, 9.58-18.4 µg/l for arsenic, and 15.1-54.3 µg/l for uranium. Water exiting the new treatment plant is currently showing nitrate levels at 4-5 mg/l, arsenic at 7-8 µg/l, and uranium at 22-24 ppb. Maximum Contaminant Levels (MCLs) are 10 mg/l for nitrate, 10 µg/l for arsenic, and 30 ppb for uranium.
The city’s efforts to reduce nitrates date from 1991, when it drilled three new wells in its long-used source, an alluvial aquifer adjacent to the Republican River. The objective was to reduce nitrate contamination in its drinking water to below the U.S. EPA Drinking Water Standard of 10 mg/l, and avoid the status of Acute Violation that the standard calls for.
While the new wells contributed 300 gal./min. input with nitrate levels at only 6 mg/l, and seemed to solve the problem, it was apparent by 1998 that blending output from the new wells with five previously existing wells could not keep the combined level below 10 mg/l during periods of peak demand. In addition, one of the major previous wells began showing nitrate levels as high as 20 mg/l, causing its 1000 gal./min. contribution to be taken out of service, and necessitating running the remaining wells for longer periods.
As the cone of influence became larger, and water was drawn from further away, nitrate levels increased further to consistent measurement at 11-11.5 mg/l. As a result, the city was placed under Administrative Order from the Nebraska Dept. of Health and Human Services Regulations and Licensure on Aug. 17, 1998, which mandated correction of nitrate levels to below 10 mg/l.
As the city moved to solve the problem via alternative water sources, it encountered difficulties and delays that caused its potential fine liability to reach tens of millions of dollars.
The city first sought an entirely new well field in an area historically low in nitrates, the Ogallala Aquifer about 10 miles away, in addition to exploration of another area of its alluvial aquifer. It also bought 2,000 acres of farmland as a non-point source. However, it soon had to sell the farmland, at a loss of $1 million, when concerns arose about contamination from its previous tenant, a military base.
Then, when the city council asked for a guarantee that new aquifer sources wouldn’t show unacceptable contaminant levels in the future, treating water from the existing well field began to emerge as the preferred option. But the need for a new clearwell, to replace one feared contaminated by a diesel spill that seeped underneath it, delayed the determination of a site for a new treatment plant until 2005, when the city could finally choose treatment of the existing water source as the solution.
Meanwhile, in late 2003, U.S. EPA had stepped in and ordered that the problem be fixed quickly to avoid heavy fines. In negotiation, the city was given relief based on full compliance with nitrate standards and simultaneous treatment for arsenic and uranium, whose maximum contaminant levels (MCLs) at that time were only under consideration, and were not yet the subject of administrative orders. As a result of the negotiation, the full compliance deadline for the city’s drinking water was established as March 31, 2006, under a Consent Decree.
In October, 2004, the city engaged W Design in a joint venture with Jacobson Helgoth Consultants to meet the deadline.
“At the desktop stage, we considered electro-dialysis reversal (EDR), coagulated aided membrane filtration, enhanced lime softening, iron-based adsorptive media, RO, and IX,” Satchell said. “We found that the capital costs of the EDR were higher than both RO and IX, and that its waste generation was much higher than with IX. Since EDR is also regarded as not as proven a technology as the others, it was eliminated from consideration. There was concern about operating costs for the filtration and softening options, and neither could handle nitrate removal, while the adsorptive media could only remove arsenic.”
The firm established RO and IX as finalist treatment options that could remove all three contaminants. In the end, IX was selected due to its low waste generation (1/4th of RO for treated water), and its lower overall operating and capital costs.
The new IX plant treats half the raw water supply, with treated water then blended back with the rest of the raw water to produce the total flow for distribution.
“Since we’re located in a fairly arid and heavily irrigated area, which has also suffered from drought recently, comparing the water lost to waste during RO and IX emerged early as a leading concern,” Dutcher said. “RO would have generated about 20-25% waste, which would be a pretty substantial loss when we peaked out at 5 mgd during the summer. By comparison, IX was to run at only 1.5-2% waste for the total flow.”
“RO also operated at a much higher pressure, 200 lbs/sq.in vs. 25 lbs/sq.in, which appeared to have resulted in a two to three-fold difference in energy costs. But despite these disadvantages, we knew RO could eliminate all contaminants, including any we found later that emerged as a concern, while IX was assumed to be only contaminant-specific, and dependent on the makeup of the resin used.”
“To answer that limitation, the IX vendor had to design a way to handle all three contaminants. When our engineer became very confident they could, we went ahead with IX. The energy cost comparison in final evaluation turned out to be not so different, but the difference in water lost to waste still remained as a decisive issue.”
Construction of the IX plant started in April 2005, and was hurried in phases to meet the March 31, 2006, deadline. It began operating in February 2006, with a Class 1 Deep Earth Injection (DEI) Well permit in hand for eventual use for IX regeneration waste disposal, and with current waste pumped to city wastewater treatment. The Administrative Order was officially lifted on October 23, 2006. A 20-year payback period is expected for plant construction and operation costs.
On-site water testing is routinely performed for nitrates and arsenic. Samples for uranium analyses are sent to an outside laboratory.
“The plant has worked flawlessly,” Dutcher said. “And the IX vendor was here last February, after a year of operation, and found the resins to be standing up fine.”
A computerized (PLC) control system operates six cation vessels containing a total of 4,242 cu.ft. of strong-acid cation exchange resin for softening, and six anion vessels containing a total of 2,352 cu.ft. of a strong-base, Type II styrenic anion resin, top-dressed with a specially graded, strong-base, acrylic anion resin.
Even though the administrative order did not include reduction of total organic carbon (TOC), the acrylic resin was included on the vendor’s advice to handle the 3 mg/l TOC expected in the supply water. The cation and anion vessels operate in married pairs on line according to demand, per Purolite’s multi-contaminant removal technology.
As flow demand decreases or increases, pairs are put on line or taken off line as needed. The cation and anion units are regenerated as needed with salt drawn from two salt brine pits located in a separate building on-site.
Treated water from the 10 ft. diameter, 21 ft. tall, 560,000 gallon capacity cation vessels and the 9.5 ft. diameter, 21 ft. tall, 579,000 gallon capacity anion vessels is blended with raw water to achieve the required contaminant compliance, while also passing requirements of the EPA Lead and Copper Rule.
Blending of raw and treated water reduces hardness from 490 mg/l to 325 mg/l. The pH of the raw water is 7.03, and the blended water is 7.3. Nine percent of the total flow is softened, and 60% of the total flow is bypassed. From raw water to plant output, total softening is about 25%.