By George C. Budd
Strategies for meeting the Safe Drinking Water Act's Stage 1 and Stage 2 Disinfection/Disinfection Byproduct (D/DBP) rules are complicated but the basic problem is easy to explain and understand: When a disinfectant is added to water, it can react with naturally occurring organic matter and other constituents to create disinfection byproducts that are not considered to be safe to drink at specified levels.
Disinfection byproducts (DBPs) can be controlled by removing natural organic material (NOM), changing the disinfectant or disinfection practices, removing disinfection byproducts, or changing reaction conditions. All of these strategies have been applied.
Strategies for removing or altering reactions with natural organic matter are likely to be the predominant methods for controlling trihalomethanes (THMs) and five regulated haloacetic acids (HAA5) to meet new requirements of the Stage 2 D/DBP Rule and will be the primary focus of this article.
Developing a Plan
Utilities need to address the following questions in the course of planning their DBP compliance strategies:
- What is the nature of precursors and how do fluctuations in concentration affect planning?
- What are the effects of new locational running annual average requirements of the Stage 2 D/DBP Rule?
- What are critical treatment profiles within the system?
- What treatment changes can be made?
- How can processes/modifications be integrated?
Understanding the variability of NOM in source water is critical for developing a plan that will assure compliance with a full range of conditions. Most of the NOM found in raw waters are vegetative decay products from terrestrial sources. Climate patterns and characteristics of a watershed have a major influence on the quantity and type of NOM. Patterns of runoff can have significant effects. For example, strong storm runoff following a drought can produce sudden increases in NOM.
Unlike terrestrial sources, which tend to be more refractory and long lasting, algae and aquatic plants produce NOM that is more biodegradable. Although their influence on base NOM levels in a water body throughout the year is less significant, aquatic sources can still be seasonally important considerations for DBP planning.
While NOM serves as the precursor material that fuels the formation of THMs and HAA5, other factors dictate the extent to which the four species of THMs and five species of HAA5 are formed:
- Temperature - Both THMs and HAA5 form to increased levels with increased temperature.
- Bromide - Bromide has a significant influence on levels of formation of different THM and HAA5 species. THM formation tends to increase with increased bromide concentration, while HAA reactions tend to shift in ways that reduce the extent of HAA5 formation.
- pH - pH also has an opposing influence on the extent of THM and HAA5 formation. More alkaline conditions increase THM formation, but decrease HAA5 formation.
Often a utility will find that decisions regarding DBP control will be driven by need to control either THMs or HAA5, depending on conditions that prevail at a given location.
Time of contact with a disinfecting agent has a profound effect on formation of DBPs such as THMs and HAA5. Effects of contact time will soon become more significant when new locational running annual average criteria of the Stage 2 D/DBP Rule require compliance with THM and HAA5 maximum contaminant levels (MCLs) at all locations in a system, including the most distant locations.
Management of detention time through modeling and modified distribution system operations can help water utilities limit excess contact time throughout a system and will become an even more important component of an overall plan for control of DBP formation as a consequence.
Some utilities have converted to the use of chloramines to provide a disinfecting residual that does not readily form THMs and HAA5 once water leaves a treatment facility, thereby limiting the effect of contact time in the system. While this approach offers significant benefits, some issues must be considered before making this type of change. A critical consideration is the need to implement strategies to limit the effects of biological nitrification, which can occur as a consequence of the ammonia content of chloraminated water. Public resistance, uncertainty about the effect of chloramines on DBPs of emerging interest, and lack of familiarity by some state drinking water regulators can also be issues in some areas of the country. In spite of these considerations, a number of utilities have successfully made this conversion.
Treatment Profiles
As an initial step in planning, it's important for utilities to fully characterize the attributes required for a treatment plant to meet all of its water quality goals. The need for such understanding derives from the fact that modifications to reduce DBP formation typically result in changes in a number of existing attributes or treatment profiles that affect other aspects of treatment. Changes need to be managed to assure that all treatment objectives within a plant continue to be met.
Basic chemical profiles typically include temperature, pH, oxidation, and NOM; varying any of these can affect not only DBP formation, but other treatment outcomes as well. Profiles that define key treatment relationships also come into play. Included among these profiles are those that directly relate to coagulation/particulate removal, disinfection, manganese, tastes and odors, and corrosion. Other profiles may be involved at some locations, depending on source water conditions. Understanding of physical treatment attributes that affect performance and general plant infrastructure are additional considerations. Within the distribution system, profiling of water chemistry, materials used for piping and other system components, disinfection residuals, detention time, and sediment accumulation are important.
Source-to-Tap Approach
DBP planning often begins at the source to identify opportunities for reducing the burden on treatment. Source reduction of NOM can be achieved through approaches such as riverbank filtration, in-lake aeration, and control of algae and aquatic plants, all of which affect the quantity and character of NOM entering a plant.
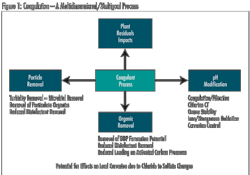
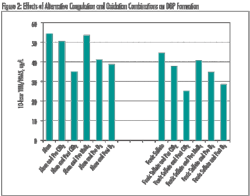
Within the treatment plant, coagulation can help reduce TOC without requiring major facility modification. In fact, regulatory requirements specify enhanced coagulation criteria for TOC removal. Increased coagulant dosage and/or decreased pH are methods to increase TOC removal, but there are pitfalls in taking a simplistic view. Increasing the coagulant dose and decreasing pH only works up to a point. Care must be taken to avoid adjustments that are unfavorable for floc formation and ultimately result in failed treatment within a plant.
While changing coagulant dose and pH consistent with particle removal goals represents one approach to consider, an alternative coagulant may provide better TOC reduction under conditions of effective particle removal. However, changing coagulation in a plant can have a significant impact on other treatment profiles and should be done carefully.
In addition to NOM removal, coagulation adjustment can affect pH, particle removal processes (clarification and filtration), disinfection effectiveness, removal of manganese, corrosion, and handling and disposal of plant residuals. In the end, the success of coagulation approaches for reducing NOM varies with water quality, nature of TOC, and ability to integrate change without creating new treatment issues. Where it is effective, this approach can significantly reduce THM and HAA5 formation.
Changes in disinfection offer another avenue for managing THM and HAA5 formation. Options include moving points of chlorination and applying alternative disinfectants such as UV radiation, ozone, and chlorine dioxide. Such changes can reduce reliance on free chlorine within a plant, thereby reducing the formation of THMs and HAA5. In cases where alternative disinfectants are applied, NOM characteristics may also be altered in ways that reduce the formation of THMs and HAA5. In addition, ozone is often applied in conjunction with biological filtration, an approach that can further reduce THM and HAA5 formation and provide other treatment benefits. These effects vary with location and testing should be performed to assess the effectiveness of the different strategies.
As in the case of coagulation modifications, disinfection changes alter disinfection and oxidation profiles in a plant and affect other treatment profiles. Effects on other aspects of treatment can be beneficial, but there is also some potential for new issues. Effects on disinfection CT need to be considered, and shifts in oxidation conditions can complicate the ability to control manganese. In some cases, new DBPs may be formed that will need to be addressed.
A number of other strategies can be incorporated within a treatment plant for improving NOM removal. Application of powdered activated carbon (PAC) is a consideration in some locations. Testing should be undertaken to confirm the degree of added removal and alternative PACs should be assessed. Adsorption by granular activated carbon (GAC) can be a very effective method of removing NOM when applied as a dedicated adsorption column with frequent regeneration. Application as media in filters can be considered, but the efficiency of GAC is not as good in this type of configuration. Ion exchange is a newer treatment approach that takes advantage of the negative charge content of NOM to achieve removal in an anion exchange approach. These systems often produce other benefits such as reduced coagulant and disinfectant demand, and can also remove other contaminants. Membrane desalting processes (nanofiltration and reverse osmosis) can be highly effective for removing NOM.
One other approach that has been applied in some situations is the use of aeration to achieve a degree of THM removal in finished water. This approach has a narrow range of application for DBPs and is not effective for HAAs, which are not easily volatilized.
There are several strategies in the distribution system that affect the formation of THMs and HAA5. One that is receiving a lot of attention is detention time and tank turnover. In addition, disinfectant demand in the system can be reduced by strategies such as flushing and corrosion control.
Integrating DBP Strategies
In-plant changes such as those that involve coagulation and points of chlorination are often good places to begin. Where appropriate, management of source water and distribution system profiles also can offer opportunities for cost effective improvement. Many situations favor a combination of strategies. Integration involves more than simply combining methods of DBP control. Effects on other aspects of treatment and capability to meet other treatment goals must be taken into consideration as well. Where added levels of treatment are necessary, more intensive treatment approaches such as ion exchange, GAC, and membrane desalting technologies can be applied. WW
About the Author:Dr. George C. Budd is part of Black & Veatch's global water business. He has more than 35 years of experience in planning, design and implementation of water treatment technologies. Dr. Budd's expertise includes the evaluation and application of conventional and emerging water treatment technologies. He served as co-author of Disinfection Alternatives for Safe Drinking Water, a book that was a compilation of input from an international group of experts.
More WaterWorld Current Issue Articles
More WaterWorld Archives Issue Articles