Solar Power Presents Bright Future For Membrane Development
An IBM research project in Saudi Arabia is looking at solar powered desalination with the aim to demonstrate a commercial-scale sea water RO plant. Robert Allen and Young-hye Na discuss the development of new membrane materials for the project designed to deal with the issues of fouling, degradation and the removal of toxins.
Many people believe water is cheap and abundant. However, one in five people on the planet do not have adequate access to safe, clean drinking water. And with over 97% of the world's water in the oceans, the availability of fresh water is severely limited. Constrained water resources today will likely become more of a concern in the future as world population growth, industrialisation and climate change continue. A sustainable supply of clean water for human use will require innovation in water purification technology, delivery and water management [1]. The 'global water crisis' has led to great interest in improved water purification technologies [2].
A nonomembrane research programme from IBM Research is looking at developing new, high performance materials for a variety of water purification applications.
This project involves partnerships on the chemicals side with Central Glass Corporation, a Japan-based high performance chemical company, on the academic side with Prof. Benny Freeman at the University of Texas – Austin, and on the membrane research and development side with King Abdulaziz City for Science and Technology (KACST), a research center in the Kingdom of Saudi Arabia. IBM has a research relationship in nanotechnology with KACST that extends beyond nanomembranes. This research partnership concentrates on three core areas:
• Photovoltaics to convert sunlight into non-polluting, abundant alternative energy though several approaches including silicon, non-silicon, and ultra-high concentrator photovoltaics
• Green chemistry to create new, sustainable materials from oil through organocatalysis
• Efficient, economical desalination to turn saltwater into fresh, through novel nanomembrane materials.
Desalination of seawater through membrane processes, specifically Reverse Osmosis (RO), is growing in importance as it combines relatively low energy with a scalable solution [3]. Membrane assembly needs to have adequate mechanical properties to withstand the pressures involved in RO operation (approximately 800 psi for sea water desalination) used to both overcome the natural osmotic pressure and to provide adequate water flux through the membrane.
The preferred solution is to use a thin film composite (TFC) membrane with three or four separate layers stacked, including the top separator layer responsible for the desalination [3]. Improved materials for RO membranes should include more effective salt removal with high flux, ability to resist fouling during long-term use, and improved chlorine tolerance. Anti-fouling coatings are a very active area of current research [4] and should resist fouling of the membrane surface from organic sources, oil, etc.
Initial entry into this field from the company was accelerated through experience in resist chemistry research. A new materials approach is discussed in this paper, a desalination membrane that directly grew out of IBM's expertise in HFA (hexafluoroalcohol) chemistry developed for 193nm resists [5].
The HFA group has very desirable characteristics: large, steric bulk for increased rigidity of the polymer (higher glass transition temperature); electron withdrawing (changing the reactivity of the part of the molecule it is attached); hydrophobicity (to repel water at neutral pH); and hydrophilic or even soluble in mildly basic water.
This "switching" of solubility in water of different pH is a highly desirable property in modern semiconductor lithography, where the exposure occurs under water (immersion lithography). The resist film needs high water repelling qualities to support the rapid scanning required for high throughput manufacturing.
In a subsequent development step, the hydrophobic film needs to dissolve in water of a high pH. The HFA-based materials perform this function better than any other materials developed to date and have thus enjoyed widespread commercial acceptance in the manufacture of advanced semiconductors.
This HFA chemistry was applied to the preparation of new RO membrane materials by the incorporation of HFA groups onto an aromatic diamine [6]. The HFA groups presumably would aid in the protection of the diamine toward oxidation, thus rendering the polymerised material more robust to chlorine attack during membrane use.
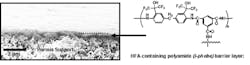
This was found to be the case as well as other performance attributes to membranes made from this novel ingredient. We were successful at incorporating the new monomer into aromatic polyamides through the interfacial polymerisation with a tri-acid chloride, a necessary first step in making a membrane. Figure 1 shows an SEM cross section of the interfacially polymerised membrane formed on a porous polysulfone support.
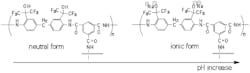
The membrane performance was found to be pH dependent, as shown in Figure 2. The salt rejection was relatively low at pH 4 (R = 85.0 %), but it increased to 96.1 % at pH 10. The water flux at pH 10 (ca. 88 LMH) was almost double that measured at pH 4 (ca. 48 LMH). Such a strong pH-dependant behavior of this membrane is due to the ionization of HFA functionalities (from (CF3)2OH to (CF3)2O-) as shown in Figure 3.
Water contact angle and XPS analyses indicated that the HFA-containing polyamide membrane is relatively hydrophobic at neutral conditions but becomes hydrophilic at basic conditions due to ionization of the HFA groups (pka = ca. 10) [6], so we refer to this group as an "ionizable hydrophobe"or "i-phobe".
Enhanced hydrophilicity and charge density of the membrane at high pH (ca. 10) contribute to increased water flux and salt rejection (Donnan exclusion), respectively.
We have subsequently found through optimisation that we can further improve the salt reject to above 99% and increase the flux at the same time. The science behind this improvement will be the subject of a future paper.
The current focus of the new membrane is scale-up and functional testing. IBM is working with KACST to demonstrate a commercial-scale seawater reverse osmosis plant within the Kingdom of Saudi Arabia, based upon the jointly developed membrane materials. We believe that this new material approach has significant advantage over conventional RO membrane polymers.
It is more robust to oxidation, has very attractive separation properties (flux, salt rejection) and can readily remove boron and arsenic salts at high pH.
PSF membranes coated with our hydrogel coatings demonstrated good fouling resistance to the organic or biomolecules, resulting in higher water flux than uncoated ultrafiltration membrane over a long period of operating time. Incorporation of anti-fouling coatings into RO membranes is currently in progress.
In summary, the discussed program has produced films that address key issued with water purification membranes such as fouling, degradation, and removal of toxins and demonstrated unique RO membrane performance with excellent combination of salt rejection and water flux.
Furthermore, the IBM RO membrane has demonstrated nearly quantitative rejection of boron and arsenic salts. We believe that materials innovation may lead to breakthrough in water purification technology.
1. National Geographic, April 2010, Special Issue.
2. R. F. Service, Science, 2006, 313, 1088.; M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Mariñas and A. M. Mayes, Nature, 2008, 452, 301.
3. R. J. Petersen, J. Membr. Sci, 1993, 83, 81.; T. Matsuura, Desalination, 2001, 134, 27.; N. Vainrot, M. S. Eisen and R. Semiat, MRS Bulletin, 2008, 33, 16.
4. Ju, H.; McCloskey, B. D.; Sagle, A. C.; Wu, Y.–H.; Kusuma, V. A.; Freeman, B. D. J. Membr. Sci. 2008, 307, 260-267.
5. R. Allen, G. Breyta, P. Brock, R. DiPietro, D. Sanders, R. Sooriyakumaran and L. Sundberg, J. Photopolymer Sci. Technol. 19(5), 569-572 (2006).
6. Y.-H. La, R. Sooriyakumaran, D.C. Miller, M. Fujiwara, Y. Terui, K. Yamanaka, B. D. McCloskey, B. D. Freeman, and R. D. Allen, J. Mater. Chem., 2010, 20 (22), 4615 - 4620
Author's note:Robert Allen and Young-hye Na are both researchers at IBM.
More Water & WasteWater International Current Issue Articles
More Water & WasteWater International Archives Issue Articles