By Richard W. Presley
Chlorination and dechlorination are vital processes in the power generation water cycle, necessitating accurate and reproducible means of measuring chlorine on site. Managers and operators looking for chlorine monitoring solutions for their specific water matrices will find several measurement methods available – including the latest developments in advanced, yet utilitarian, automated titration.
Measuring chlorine
Close scrutiny of residual chlorine is required for:
• Biogrowth control – Warm, moderately alkaline cooling tower water presents ideal microbial growth conditions. The inorganic and organic phosphates added for corrosion control support the growth of algae, fungi, and bacteria that can contribute to corrosion, wood deterioration, and reduced heat exchanger efficiency. Periodic chlorine shock treatment kills resistant microorganisms while lower-level treatments limit growth. A total residual chlorine (TRC) concentration of 2.5 mg/L will kill adult zebra mussels in 10-15 days while 0.5 mg/L inhibits their growth.
• RO membrane protection – Reverse Osmosis (RO) treatment utilizes semi-permeable membranes to remove dissolved solids and microbial contaminants prior to boiler feed. Chlorine can degrade these membranes, and monitoring chlorine in the low-µg/L range after dechlorination guards against membrane damage.
• Wastewater discharge compliance – Federal and state agencies typically impose MCLs (maximum contaminant levels) – the primary enforceable standards – between 0.010 and 0.30 mg/L total chlorine in cooling water discharge to lakes, rivers, and marine estuaries. Renewed permits are seeing even lower allowable chlorine concentrations.
Analysis alternatives
The direct colorimetric method uses DPD (N,N-diethyl-p-phenylenediamine) indicator and instruments that are pre-calibrated for direct readout of free chlorine, total chlorine and monochloramine concentrations. Results and detection limits depend on purity and stability of the DPD indicator, buffer, and potassium iodide reagents; the presence of positive interferences including bromine, hydrogen peroxide, ozone and iodine and sample particulates and color; the optical quality of the sample cell; and instrument optical system specifications.
Chlorine measurements by titration with ferrous ammonium sulfate (FAS)-DPD titrant and iodometric titrations with starch indicator rely on visual (color) endpoints and are subject to similar oxidizing agent interferences as the DPD colorimetric method. These tests also suffer from variability associated with classical glass burettes, individual operator skill, unstable reagents, and manual endpoint determination.
Most analysis references, including Standard Methods for the Examination of Water and Wastewater – published by the American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF) – define amperometric titration as the standard methodology for determination of free or combined chlorine. This technique utilizes an amperometric probe with a constant applied potential, and the measured current is proportional to the concentration of the oxidation-reduction (redox) species active in the sample. Adding titrant results in irreversible reaction that lowers the concentration of the active redox species and reduces current flow, which reaches zero at the equivalence ("dead-stop") point. The volume of titrant delivered, along with the sample volume, the normality of the titrant, and the stoichiometry of the half reactions, determine sample concentration. This methodology is less affected by common oxidizing agents, temperature variations, turbidity, and color than other chlorine-measurement procedures.
Autotitration approach
Autotitration facilitates amperometric measurement for plant operators. As such, today's automated titrators – sophisticated instruments that deliver precise and accurate titrant and sample volumes – improve accuracy, precision, and method detection limit (MDL) compared to manually performed amperometric titration. However, these general-purpose tools often require extensive method programming and a sound knowledge of analytical chemistry.
The recently introduced AutoCAT™ 9000 Automated Chlorine Amperometric Titrator from Hach Company targets reliable trace chlorine measurement by operators without analytical chemistry backgrounds and extensive analytical instrumentation experience. It incorporates a potentiostat that controls the potential applied to the amperometric probe to ±0.1 µV. (Other autotitrators apply a constant current to an electrode to monitor the resulting potential, resulting in a curve that reflects an inflection point titration instead of a dead-stop titration; such a method modification may be unsuitable for regulatory reporting purposes.) The Hach chlorine autotitrator includes elements that target application in power generation water quality management:
• Embedded chlorine amperometric titration methods and titrant standardization – Instrument software contains pre-programmed methods for determining chlorine, chlorine dioxide, chlorite and sulfite, which often is used in boiler feed water for dissolved oxygen control and wastewater discharge for dechlorination (see Figure 1).
• High-increment stepper motor that delivers the smallest titrant volume increment for a given volume burette cylinder – The instrument's 18,000-step stepper-motor, in conjunction with the standard 5-ml burette, achieves a smallest deliverable volume increment of 0.3 µl. At a sample volume of 200 ml and standard 5.64 mN PAO titrant, this delivery is equivalent to a 0.3-µg/L chlorine increment.
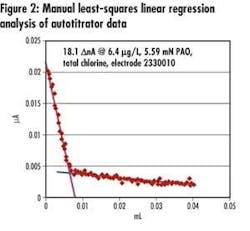
• Automated endpoint determination and results calculation – The instrument pairs titrant volume measurements with current output readings and, using embedded endpoint determination algorithm and equations, identifies the 'dead-stop' endpoint, and final chlorine concentration (see Figure 2).
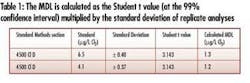
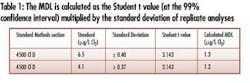
• Demonstrable detection sensitivity – Analysis according to Title 40 CFR Part 136, Appendix B, determined an evaluation instrument MDL to be 1.2 µg/L (see Table 1). This detection limit is suitable for facilities required to meet NPDES discharge limits in the low-µg/L range.
• Real-time graphical display of titration curve – Instrument display allows the analyst to view the titration progress, abort titrations whenever a problem is noted, or adjust method parameters such as volume increment or titrant pre-dose if needed.
• Stand-alone operation – The instrument doesn't require a separate personal computer for operation.
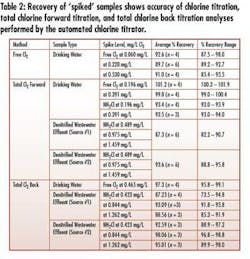
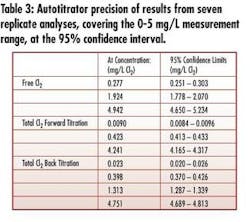
• Wide-range (0-5 mg/L) measurement and precision – The instrument measures very low concentration ranges typical of critical RO feed and higher concentrations typical in disinfection for biogrowth control – without sample dilution and using the same normality of titrant (see Tables 2&3).
• Ability to archive and print out the titration curve, user identification, sample, titrant standardization, time and date – Documentation is beneficial particularly when several operators, over different shifts, use the instrument. Hardcopy printout provides the traceability mandated for National Environmental Laboratory Accreditation Program (NELAP)-certified laboratories.
• A biamperometric system – Use of dual platinum pins and no reference electrode results in reversed polarity of the potential with each titration, an action that automatically strips anodic or cathodic electrochemical depositions.
Conclusion
Even automated amperometric titrators require some care and feeding as well as attention to choice of glassware, appropriate instrument settings, and proper laboratory methodology to avoid sample carryover and reagent contamination. Some power plants have found support from the plant laboratory staff helps effect a practical, in-house chlorine monitoring program. With proper training, operators can complete reliable chlorine measurement – even at trace levels – throughout the water cycle, over all shifts, to protect equipment, prevent downtime and costly corrective maintenance, and maintain discharge regulatory compliance.
About the author:
Richard W. Presley received his master's degree in applied chemistry from New Mexico Highlands University and applies his electrochemistry expertise toward research and development, product application and product management at Hach Company. He was a key contributor in developing the company's chlorine-dedicated automated titrator for use in water and wastewater treatment and industrial water applications. He can be contacted 800-227-4224 or [email protected]