Jurek Patoczka, PhD, PE
The New Jersey Dept. of Environmental Protection has started to enforce strict phosphorus limits on dischargers in northern watersheds, where impairment of water quality was declared. Several permits (and permit renewals) issued recently imposed total phosphorus limit as low as 0.1 mg/L. Both municipal and industrial direct dischargers located in the watersheds are expected to be affected.
The paper reports on jar tests results and full-scale pilot plant trials aimed at optimizing chemical addition for phosphorus removal to low levels. The jar tests were performed on samples collected from several wastewater treatment plants. Of particular interest were:
• Comparison of alum and ferric chloride in removing residual phosphorus to low levels,
• Impact of staged (multipoint) chemical addition on removal effectiveness,
• Impact of pH on effectiveness of trace phosphorus removal.
Subsequently, full-scale trials withmultistage alum addition commenced at a treatment facility equipped with primary clarification, activated sludge and traveling bridge sand filters.
Methodology
Chemical addition tests were performed by adding alum or ferric chloride stock solutions to 1-liter beakers, flocculating (slow mixing) samples for five minutes and settling for 30 minutes. Any necessary pH adjustments were made with a solution of caustic or sulfuric acid prior to initiating the settling. Where indicated, filtered samples were obtained by filtration through GE Osmonics MicronSep cellulosic membrane filters with a nominal pore size of 0.45 µm. Where indicated, settling was aided by addition of Pollu-Treat 116 polymer distributed by Pollu-Tech Inc. at the end of the flocculation period and accompanied by flash mixing for 30 seconds followed by three minutes of additional flocculation time. Total phosphorus (tP) was analyzed by EPA Method 365.1.
Comparing Alum & Ferric
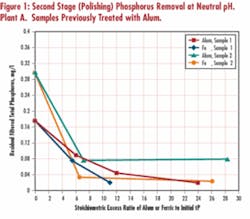
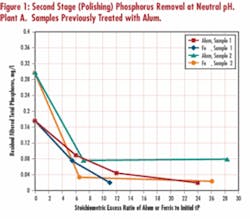
Side-by-side tests on samples from several wastewater plants demonstrated that ferric was typically more effective than alum at the same stoichiometric excess. Tests on secondary effluent from Plant A, where alum was previously added to mixed liquor at full scale, indicated ferric lowered the filtered phosphorus concentration to below 0.04 mg/L at a lower stoichiometric excess ratio than alum (see Figure 1). Similar results were obtained with Plant B samples (no chemical addition in full scale), with ferric achieving lower ultimate phosphorus residual than alum. This is contrary to theoretical phosphate salts solubility considerations at neutral pH range, which seem to favor use of alum. Factors such as presence of phosphorus in forms other than orthophosphate, complexing agents and elements of adsorption removal mechanism, however, limit practical applicability of straight chemical solubility calculations.
Optimal Addition Point
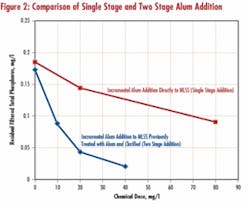
A mixed liquor sample from Plant A (alum previously added to mixed liquor at full scale) was dosed with additional alum and results contrasted with effectiveness of similar alum dosages applied to the clarified sample. The results demonstrate an additional, relatively small alum dose, when applied to clarifier effluent, is much more effective than the same incremental dose applied to the mixed liquor alone (see Figure 2). While the advantage of dual point chemical addition was previously recognized, these results confirm it. This is consistent with incorporating into the mechanism of phosphate removal the sorption of orthophosphate on Al(OH)3, as postulated in a 1997 study. Actually, a mixture of various hydrous aluminum oxides and hydroxyphosphates is probably involved. This is further corroborated with results from this work, where phosphorus removal in jar tests from various sludge processing side streams correlated well when modeled with Freundlich adsorption isotherms. An adsorption-type mechanism of phosphorus removal would predict enhanced efficacy of two (or multi)-point chemical addition, as such staging introduces elements of counter-flow operation.
Effect of pH
Optimum pH for phosphorus removal by alum or ferric salts has been reported to vary considerably within the moderate pH range, based on theoretical considerations and actual results. Test results on the primary clarifier effluent (Plant A) indicate increased removal with alum is favored by more acidic pH, while no pH dependency was apparent for ferric in the neutral range of pH tested. Tests on a secondary effluent (Plant B) confirmed no noted pH dependency for either alum or ferric. Based on these study results the literature data, it appears pH effects are site-specific and often aren’t expected to be pronounced in the neutral pH range practicable for wastewater treatment.
Full Scale Trials
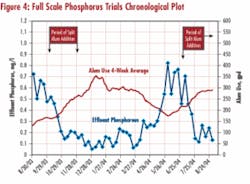
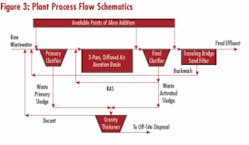
Subsequently, multistage addition was implemented at a 3-mgd plant with a three-pass activated sludge system preceded by primary clarification and followed by sand filters (see Figure 3). Aeration in the first pass is severely curtailed to induce denitrification (effluent NO3-N averages 6 mg/L). The facility has averaged 0.6 mg/L total phosphorus in the effluent (1 mg/L permit limit) by alum addition to secondary clarifiers, at an average dose of 45 mg/L. Attempts were made at inducing biological phosphorus uptake by moving alum addition to the primary clarifier effluent, but phosphorus, release couldn’t be induced. This failure was attributed to a low BOD5/P ratio. The primary objective of the full-scale trial was to determine if available facilities, including the newly refurbished traveling bridge shallow bed sand filters, were capable of achieving the future permit limit of 0.1 mg/L.
Following minor modifications to the chemical addition system, a second alum dose of 15 mg/L was introduced in November 2003 to the secondary clarifier effluent. The effluent tP concentration decreased to 0.15 mg/L, confirming efficiency of multistage addition that was observed in the jar tests. Subsequently, alum addition to the secondary effluent was further increased, and the effluent phosphorus concentration averaged below 0.1 mg/L at the end of 2003 and beginning of 2004 (see Figure 4). This result indicated there's a good possibility, with a high enough alum dose, the plant might be able to meet monthly average limit of 0.1 mg/L with existing facilities (traveling bridge sand filters). So, alum addition was gradually reduced and than increased again, prompting an expected response in the effluent phosphorus concentration.
No adverse impact on sand filter operations has been noted to date, except for a minor increase of backwashing frequency. The continuing trials may include use of ferric at the secondary addition point and polymer addition to enhance solids capture.
Conclusion
1. Multistage chemical addition is more effective in phosphorus removal to low levels as compared to a single-point application of the same dose, with elements of adsorption-type removal mechanism evident from the jar tests.
2. Precipitation with ferric appears to achieve lower residual phosphorus than alum (at trace level).
3. pH does not have a major impact on phosphorus precipitation efficiency with alum or ferric in the moderate pH range.
4. Full scale trials indicate feasibility of achieving 0.1 mg/L limit with the existing shallow bed filters.
About the Author:
Jurek Patoczka is a process design specialist at Hatch Mott MacDonald, of Millburn, NJ, where he's worked since 1989. He holds a doctorate in environmental engineering from Vanderbilt University and a master's degree in chemical engineering from Krakow Technical University (Poland). He's a registered professional engineer. For references to this article or other information, contact him at 973-912-2541 or email: [email protected].