By Dave Anderson
Desalination is becoming a very popular alternative to the use of surface water to meet the world’s demands for a fresh water supply. Key to the efficiency and affordability of desalination plants is the strategic use of liquid analysis, particularly conductivity and pH measurements, to monitor water chemistry throughout the process.
The desalination process is broken into two primary technologies:
Power consumption is a critical consideration. Thermal Distillation is favored in places with plentiful power, such as the Middle East, while membrane technologies are most predominant in the West. Approximately 50 percent of the desalination capacity uses RO technology while 33 percent employs MSF.
Thermal distillation works much like the natural water cycle. The water vapor from heated salt water is condensed to produce desalinated water. The water vapor can be used as a source of thermal energy to heat more of the incoming salt water. In MSF, the hot brine passes to the first stage. The pressure in the first stage is below the saturation pressure of the heated brine. A portion of the brine flashes to steam.
The heat of vaporization required to boil the brine comes from the hot brine itself. As a result, the brine cools to the boiling point, but no further boiling occurs because there is no more heat input. The steam is condensed on a bank of tubes and collects as distillate. The cooling water (tube side) in the condenser is incoming seawater. Thus, the condensing vapor preheats the seawater.
The brine then passes to the next stage, which is at a lower pressure than the previous stage, and the process continues. Boiling of the same water in multiple vessels occurs, each time at a different pressure and temperature. These multiple stages reduce the amount of energy required for distillation and increase the yield of fresh water. Unfortunately, it also contributes to one of the key problems in thermal distillation — the formation of scale.
Scaling Problem
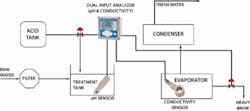
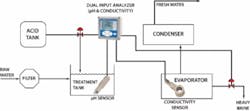
In a typical distillation, the raw seawater is filtered to remove solids and then combined with acid in a treatment tank. The treated seawater is heated in an evaporator where much of the scaling can occur. The resulting vapor is collected and re-condensed in the condenser as fresh water and the salt is removed as brine.
In the evaporator, there are two distinct scaling problems. The first is with the calcium carbonate and magnesium oxide scale formed under higher pH conditions. Lowering the pH of the feedwater to less than 5.7 pH can minimize calcium carbonate and magnesium hydroxide scaling. This is commonly achieved by adding citric acid, ferric chloride, or sulfuric acid. Too much acid, however, will result in corrosion; therefore precise pH control must be maintained. Typically a pH sensor will be placed in the treatment tank to help control this process.
A second scaling problem occurs when the feed solution becomes saturated with calcium sulfate. Adjusting the pH has no impact, so a portion of the heavy brine must be removed from the evaporator. Conductivity sensors are used to monitor the saturation point. Although conductivity doesn’t provide a specific indication of the concentration of calcium sulfate, it does correlate to the level of all dissolved solids. Inductive conductivity sensors are immune to sensor fouling in this highly corrosive environment.
Membrane Problems
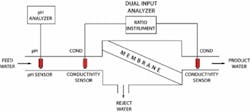
In membrane-based reverse osmosis, pressure forces water through the membrane to reject most solute ions and molecules while allowing water of very low mineral content to pass through. Passing water through the semi-permeable membrane under pressure means that the concentrated brine is rejected, while the desalinated water passes through the membrane.
RO membranes have the capacity of rejecting up to 99 percent of all ionic solids, but special attention must be given to the amount of pressure applied. Higher pressures increase the flux of water through the membrane, which is good, as it also leads to better permeate quality. The concentration of dissolved solids on the feedwater side of the membrane increases with increased pressure, causing the brine to escalate. This condition results in fouling or build up on the membranes, increasing maintenance and shortening membrane life.
The critical element in reverse osmosis desalination is the condition of the semi-permeable membrane since this membrane can represent a large capital investment. Frequent replacement is costly and time-intensive. The principal problem is fouling caused when membrane pores are clogged by salts or obstructed by suspended particles. As a result, pre-treatment is an important step usually consisting of fine filtration, suspended solids removal, pH adjustment and adding scale inhibitors to control the scaling during this portion of the process.
Conductivity is used to monitor the efficiency of not only the RO process but also the membrane. Calculations are done by dividing the amount of dissolved solids content of the product water by the volume of the feedwater. For example a new plant will show between 2-5 percent passage and 95-98 percent rejection. Over time, operators will see a decrease in rejection indicated by the conductivity analyzer, which will warn them either by alarm or SCADA detection that the membranes may need to be maintained or replaced.
pH is monitored to insure the semi-permeable membranes are not prone to degradation from exposure to alkaline water. Such degradation can cause a loss of efficiency and require expensive membrane replacement. In addition, continuously controlling raw water inlet to 5.5 pH coming into the vessels or RO skid allows operators to control any possible scaling on the sample side of the membrane.
Post Treatment
Once the seawater has been through either the distillation process or reverse osmosis, every desalination plant has to address post treatment. This process typically includes decarbonation/degasification for carbon dioxide and hydrogen sulfide removal. The water trickles from the top of the silo while air is blown up from the bottom. pH and hardness adjustment for corrosion control are very important in this part of the treatment process because the permeate water is very aggressive. Therefore, the water is analyzed for both pH and turbidity after it passes from the RO membrane, but before it enters decarbonation or degasification.
In an RO application, the permeate for brackish water is approximately 10-20 mg/L Total Dissolved Solids (TDS). For saltwater it’s 400 mg/L TDS; the alkalinity is low and the pH is roughly 1-1.5 pH units below feed value. In thermal distillation, the total dissolved solids are even lower and the water is much more aggressive. In brackish water, reverse osmosis permeate is virtually demineralized so a portion of the raw water from the front of the plant is blended with the permeate leaving the building to blend and stabilize the water. This decreases chemical costs to harden the water and prevents corrosion of the pipes and distribution system. The final step is disinfection via chlorination. The water leaving the plant is analyzed for chlorine, pH, and calcium concentration to determine lime concentration and meet regulatory discharge regulations (or requirements).
The figure on the previous page shows the distribution of all types of analyzers throughout the desalination process. As can be seen, desalination facilities rely extensively on on-line analytical equipment including pH, ORP, conductivity, turbidity, and chlorine to monitor many crucial steps throughout the process. This is done to maintain the most vital element of desalination — water chemistry. WW
About the Author:
Dave Anderson is Life Sciences Industry Manager at Emerson Process Management, Rosemount Analytical Liquid. He has more than 17 years in the industry and can be reached at 949-757-8500. For more information, see www.raihome.com.