While the debate on perchlorate contamination continues, many municipalities are faced with the responsibility of removing perchlorate from their drinking water systems. Although some early installations of ion exchange resins for perchlorate removal suffered from relatively high treatment costs, the technology has been evolving in recent years. This article discusses the evolution of ion exchange and its companion technologies for perchlorate removal from drinking water.
An ion exchange resin can be visualized as a three-dimensional hydrocarbon network to which a large number of ionizable groups (also called functional groups) are chemically attached. Depending upon the ionizable group, the ion exchange resin can be classified as a cation exchange resin to remove cations or an anion exchange resin to remove anions (amine function group).
During operation, contaminated water is pumped through the vessel(s) that hold the ion exchange resin. The perchlorate ion in the water is attracted to the resin by an opposing charge mechanism. As it attaches to the resin, it displaces a chloride ion. Over time, the resin reaches its adsorption capacity and becomes saturated with perchlorate and competing ions, and must be either disposed of or regenerated to ensure ongoing removal efficiency.
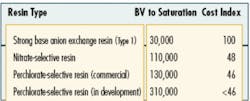
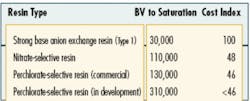
Complicating factors associated with the use of ion exchange are anions, such as nitrate and sulfate ions, present in the water at much higher concentrations than the perchlorate anion. When water is pumped through the ion exchange system, these other anions compete with perchlorate for the available anion exchange sites on the resin. As a result, a conventional anion exchange resin has a relatively low perchlorate selectivity, limiting its overall perchlorate-removal capacity. This factor was to blame for the relatively high treatment costs associated with early versions of ion exchange systems that were deployed for perchlorate removal.
In recent years, perchlorate removal using ion exchange technology has advanced significantly. Several advanced ion exchange resins that demonstrate improved perchlorate selectivity and adsorption capacity have been developed. Such advanced resins have helped to dramatically decrease the overall treatment costs associated with this technology and more are in development today.
Design Choices
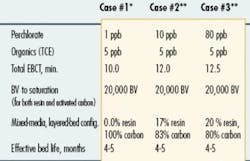
Typically two types of ion exchange systems are available for perchlorate removal: a regenerable system that relies on either a moving-bed or fixed-bed design, and a non-regenerable system that relies on a single-use, fixed-bed design. System selection is made on the basis of site-specific factors such as water chemistry, required installation speed, ease of operation, and overall treatment cost as well as the end treatment criteria and prevailing drinking water compliance standards.
Capital and operating costs associated with an ion exchange system will vary depending on site-specific factors that include the volume of water being treated and the purity levels required, the nature and concentration of the contaminants, the specific system design, and the type of resin regeneration (or periodic disposal) that is employed. In general, operational costs are expressed in terms of $/acre foot or $/1000 gallons of water treated.
Single-use, fixed-bed non-regenerable system design
Single-use, fixed-bed systems offer the advantage of simplicity. They are modular in design and, typically, can be quickly delivered and installed. For example, an entire system loaded with resin beads can often be delivered in just six weeks and operational within a week of delivery. With single-use systems, the exhausted resin is periodically replaced with virgin resin and the spent resin is removed from the facility and sent for incineration. The incineration of spent ion exchange resin, with certificates to verify destruction, ensures absolute mitigation of the perchlorate problem for the system operator.
Operation of such systems is simple and the capital investment required is relatively low. Conversely, operating costs associated with the periodic replacement and disposal of the resin can be relatively high. In general, the operating costs associated with non-regenerable ion exchange systems range from $80/acre-foot to $260/acre-foot.
Moving-bed regenerable system design
A truly continuous ion exchange system uses 20-30 ion exchange beds mounted on a slowly rotating carousel. The rotation of the carousel moves the beds slowly through the required sequence of operations which normally includes adsorption, regeneration, rinse, and displacement. These operations are on-going simultaneously as the carousel rotates. The different fluids are distributed through a 2-in-1 valve with 20 or 30 ports allowing for either concurrent or countercurrent flows in all operations. Rotation is provided by a fractional horsepower motor with low energy consumption. Essentially, there is only one moving part which typically rotates 2-6 times per day. Valves are limited to 5 or 6, unlike a fixed bed regenerable system which requires 30 or more valves or automated switches.
The initial capital investment required for a regenerable system is higher than for a fixed-bed system and operating costs range from $100/acre-foot to $260/acre-foot. Regenerable systems, however, offer the advantage of small footprints, high regeneration efficiencies, and automated operation.
Fixed-bed regenerable system design
In a regenerable fixed-bed system, anion resin is packed into fixed beds and is used to remove perchlorate from water that is pumped through the treatment vessel. Unlike a non-regenerable system where the saturated resin is periodically removed for disposal and replaced, the perchlorate-saturated resin in a regenerable system is periodically removed from the vessel and regenerated either on or off-site. The regenerated resin is then returned to the fixed-bed system and reused. Such an approach can potentially reduce the final treatment cost by 50 to 70% compared to a non-regenerable system using the same resin.
In 2001, researchers at Oak Ridge National Laboratories (ORNL; www.ornl.gov) developed and patented a special process using a unique regeneration solution to regenerate the ion exchange resin used in perchlorate-removal systems. The ORNL technique uses the FeCl4(-) anion (produced by Fe(+3)+4 Cl(-) <−> FeCl4(-) reaction) rather than the Cl(-) anion (in a conventional brine regeneration solution) to displace adsorbed perchlorate and other ions and regenerate the resin.
Since the FeCl4(-) ion has a higher affinity than the perchlorate ion for the amine functional groups on the anion exchange resin, it can effectively and easily regenerate anion exchange resins - including today’s advanced perchlorate-selective resins - using relatively small amounts of regeneration solution. The availability of such a high-efficiency regeneration process makes today’s regenerable fixed-bed systems a cost-effective option for removing perchlorate from drinking water.
Final thoughts
The perchlorate issue is not without controversy. Public awareness is growing as more and more drinking water sources are found to be contaminated and regulators debate what the appropriate regulatory thresholds should be. Since the first ion exchange perchlorate treatment system for drinking water production began operation in 2000, ion exchange has become a proven and well-accepted solution for removing perchlorate from drinking water. Today, several ion exchange technologies have been used and more are under development.
The treatment costs using resin have decreased significantly since the first system began operation and this trend is expected to continue, especially with the development of regenerable fixed-bed systems.
About the Authors:
Chen-Chou Chiang is a senior research fellow with Calgon Carbon Corp. in Pittsburgh, PA. He has more than 20 years industry experience in activated carbon applications, adsorption, ion exchange, chromatographic separation, catalysis and process research & development. Currently he is the lead investigator in perchlorate removal related technologies. Kim Megonnell is an Industry Manager for Calgon. She is responsible for assessing, defining and building market strategies in new and existing markets.