by Sue Rivera
The prevalence of membrane filtration is rapidly growing in both the municipal and industrial water sectors. As membrane chemistries improve, so do operational capacities and process requirements. Two main activities that contribute to membrane efficiency and lifetime are membrane pretreatment and membrane maintenance. Maintenance may be further differentiated into periodic backwashing and cleaning to remove particulates and biological/organic fouling.
This article focuses on a technology - chlorine-based mixed-oxidant solution (MOS) - that assists in oxidizing and removing organics during membrane pretreatment and then acts as a disinfectant to remove biofoulants from membrane surfaces. Operational experience shows that pretreatment oxidation often eliminates the need for frequent backwashing while periodic or continuous mixed-oxidant feed prevents biofilm regrowth on ultrafiltration (UF) membranes. Studies indicate that mixed oxidants will remove biofilm from cellulose acetate and polyamide membranes as well.
Site Examples
In 2000, an SAL-80 mixed-oxidant on-site generator from Miox Corp. was installed at the water plant in Martindale, TX. Martindale’s source water is classified as ground water under the influence of surface water. After the water is pumped from the well, mixed oxidants are dosed prior to hydropneumatic tanks. The water then goes through a pre-filter to a Koch UF system and then into ground storage tanks. The UF system is backwashed every two hours, the maximum recommended time by the manufacturer. Because of MOS’s ability to remove biofoulants, the filters are physically cleaned only once every six months on average. The operators credit the infrequent cleaning regimen to the ability of mixed oxidants to remove biofilm and other organic contaminants in the system. The initial MOS dose of 1.5 to 1.8 mg/L FAC is sufficient to retain a 1.4 mg/L residual throughout the entire distribution system.
In 2004, a MIOX-502 mixed-oxidant on-site generator was installed in Harvey, ND, for iron and manganese oxidation and for residual disinfection. Mixed oxidants effectively oxidize the iron and manganese prior to removal through a granular sand filter. The water is then dechlorinated before passage through a nanofiltration membrane and then rechlorinated for final disinfection.
A different site in Texas uses MOS to keep a UF pre-filter clean. Per manufacturer recommendations, however, chlorine is removed prior to the next filter in the treatment train, a polyamide reverse osmosis (PA RO) filter.
Do oxidants really degrade PA RO membrane filters?
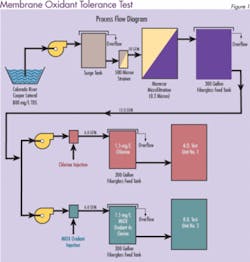
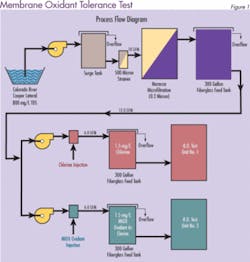
Most PA RO manufacturers do not recommend the use of chlorine. However, a long-term test evaluation performed by Separations Systems Inc. at the U.S. Bureau of Reclamation Yuma Desalting Plant Water Quality Improvement Center (Yuma, AZ) indicates that in the presence of low ferrous iron (< 0.01 mg/L), neither chlorine nor MOS appreciably degrades PA RO membranes. Three commercially available spiral-wound PA RO membranes (Dow/FilmTec, Hydranautics, and Koch/Fluid Systems) were tested for their relative tolerance to MOS and chlorine. In addition, an oxidant resistant cellulose triacetate membrane (CTA-Osmotek) was used as a control.
Initially, one train contained 1.5 mg/L free chlorine and the other contained 1.5 mg/L MIOX oxidant. To accelerate membrane deterioration, oxidant levels were increased periodically during the test from 1.5 to 5.0, 10 and 20 mg/L. Pressure, temperature, pH, FAC, conductivity, flow and recovery were measured daily and used to evaluate system performance. Adjustments were made as necessary to maintain experimental parameters.
The results were surprising. The membrane resistance to both disinfectants was much greater than anticipated. Figure 2 shows the percentage of salt that was able to pass through the membrane when mixed oxidants were used as the disinfectant. An increase in salt passage through the membrane (decrease in salt rejection) is an indicator of oxidative membrane degradation. Similar results were obtained for chlorine (data not shown). Stability of the membranes was attributed to low ferrous iron (< 0.01 mg/L) in the feed water. The test concluded that both MOS and chlorine are acceptable for use with commercial polyamide membranes.
Do oxidants degrade cellulose acetate membrane filters?
At the Orange County Water District in California, Dr. Don Phipps, Chemist and Microbiologist, studied the effects of MOS and of sodium hypochlorite on the removal of Pseudomonas putida biofilms on cellulose acetate RO membranes. A higher initial oxidant demand of 17 mg/L with mixed oxidants versus only 9 mg/L with chlorine was reported. However, mixed oxidants destroyed the polysaccharide biofilm substrate, while chlorine did not. DAPI staining showed no intact DNA remaining on membranes treated with MOS. The authors were impressed by the removal of the substrate itself, the speed with which these events occurred once the proper dosage was reached, and the ability of mixed oxidants to prevent biofilm regrowth compared to FAC as bleach. Real time video collection showed removal of biofilm at 4 hours and 40 min. Nearly complete removal was accomplished by 5h 40 min.
About the Author:
Susan B. Rivera, Ph.D. is a biochemist at MIOX Corporation, headquartered in Albuquerque, NM. MIOX Corporation offers both mixed-oxidant and hypochlorite on-site generation systems for a variety of worldwide applications.