By Ted Huck
Carbon-steel water storage tanks are a common component to many municipal water systems. They come in two general configurations – the ground storage tank and the elevated water tower. As with any steel structure, corrosion is an ever present concern. The effects of corrosion on storage tanks include premature failures and disruptions in service during repairs. Cathodic protection (CP), however, stops the corrosion reaction when properly applied. This article provides a brief overview of the corrosion reaction and the role of cathodic protection for both ground storage tanks and elevated water storage towers.
Corrosion Basics
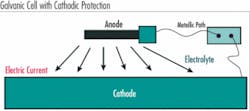
Steel naturally reacts with water and oxygen releasing energy and returning to its more stable chemical state, iron oxide. When this oxidation/reduction reaction occurs to steel we call it corrosion. To understand and prevent corrosion, it is important to understand the reaction process. There are four key elements that are required for corrosion of steel to occur and when combined together they are referred to as a galvanic cell. The galvanic cell requires:
- Anode – the anode is where corrosion occurs and is characterized by a lower electronegative potential than the cathode
- Cathode – the cathode is where current flows from the anode completing the corrosion reaction and is at a higher electro-potential than the anode
- Metallic path – the metallic path allows current to flow and completes the reaction circuit
- Electrolyte – the electrolyte is a conductive environment that supplies the necessary reactants for corrosion to occur
Unfortunately, three of the four components typically exist within any steel structure.
Steel, at the micro-level, is not entirely homogeneous and as such there are minor differences in electrical potential that occur naturally from one area to another along the surface of a steel structure. Thus the same piece of steel can provide both the anode and the cathode. And since steel is a conductor of electricity, the adjacent cathodic and anodic steel regions inherently have a metallic path. Thus, all that is needed is a suitable electrolyte – water, soil, even a thin film of condensation is all that is needed for the corrosion process to occur.
Where current leaves the metallic structure, corrosion occurs resulting in iron from the steel structure reacting with water and oxygen to form iron oxide. Where the current returns to the metallic structure, there is no corrosion reaction but instead hydrogen molecules form at the surface protecting the steel from the environment.
The rate of the corrosion reaction is a function of current flow with one amp of current consuming approximately 20 pounds of steel in one year. Thus milliamps of electrical current over several years can have a devastating impact on the tank bottoms, side walls and the roof of steel storage tanks. Left unprotected, storage tanks can fail quickly as the result of these corrosion reactions.
Internal Corrosion
Tank designers use coatings as a primary defense against corrosion on the internal wetted surfaces of water storage tanks. Coating systems, however, are less than perfect and they degrade over time. Corrosion is a pervasive phenomenon and wherever there are voids, holidays or coating failures--the galvanic corrosion cell will rush into action and continue unabated unless addressed. The common means of supplementing the coating system is to install a cathodic protection system inside the storage tank.
Cathodic protection provides electrical current to those areas not isolated from the environment by virtue of being coated, and therefore stops the corrosion cycle. Therefore, the combination of coating and CP work in concert to arrest the corrosion reaction when properly applied and maintained.
There are two basic types of cathodic protection systems – the first type is the galvanic system, which relies on anodes made from metals that are inherently more electronegative than steel. Zinc and Magnesium are common anode materials used in water storage tanks. The second type is an impressed current system where longer-lasting anode materials can be used in conjunction with an external power supply, or rectifier, which converts AC power to DC current. Alternative energy sources are also available including solar power panels connected to DC batteries, which then supply current to the anode system.
A variety of configurations are available for suspending, hanging or attaching anodes to the tank for internal protection. Several factors influence the CP system design including water quality, tank coating quality, desired anode system design life, the availability of power, freeze protection/potential and more.
null
External Corrosion
It’s not only the internal wetted surfaces that are susceptible to corrosion. External tank bottoms on ring wall foundations can corrode from contact with the ground base. For these applications, accepted engineering design practice is to utilize impressed current CP systems to protect the tank bottom. Impressed current systems typically have a longer design life than galvanic systems, and it if properly maintained, don’t need to be replaced as sacrificial galvanic anodes under tanks do.
As with internal tank CP systems, there are several configurations for external tank bottom CP systems. One common approach is the use of linear anodes in concentric rings. The principal advantage of the linear system is that everything under the tank is factory assembled and tested prior to installation and the only installation effort is to lay the anode assemblies in accordance with the design drawings and installation instructions. This provides for an exceptionally simple installation while assuring the highest system reliability; installation costs are minimal.
There are also several means to apply CP to existing tank bottoms, Variables such as the structure of the base of the tank, barriers between the soil and the tank bottom, and nearby structures help engineers to determine which CP system to use.
Testing CP Systems
Testing requirements vary with the application – for internal wetted surfaces, a portable reference electrode can be suspended from the roof of the tank to take potential measurements or permanent reference electrodes can be installed at select locations. For tank bottoms systems, it is critical that provisions for testing be installed with the anode system. Once the tank is erected, making accurate potential measurements at various locations under the tank can only be accomplished if reference electrodes have previously been installed below the tank.
Typically, copper-copper sulfate reference electrodes are installed in strategic locations underneath the tank. These reference electrodes are often mistakenly called “permanent.” However, over time the copper-copper sulfate solution becomes contaminated and ceases to provide accurate information. Once a baseline for performance is established over a sufficient operating period, maintaining the appropriate current output to achieve NACE criteria is all that is required.
In some cases, clients may also specify dual reference electrodes such as both zinc and copper-copper sulfate. While the zinc reference electrodes are not as consistent, they provide a much longer operating life and can be calibrated against the copper-copper sulfate electrodes.
In addition to the fixed reference electrodes, it is also common to provide a reference electrode tube/conduit underneath the tank bottom to allow sliding of a calibrated reference electrode through the monitoring tube to take potential readings. These can also function as leak detection tubes.
Annual testing/inspection of the tank cathodic protection systems by a qualified NACE CP Level 1 or higher technician is typically recommended. For impressed current systems, monthly rectifier checks should be performed by plant maintenance to assure that the rectifier is on and that the voltage and current outputs remain stable.
Water tanks are a critical investment for municipal water systems, and the effective application of cathodic protection is an important tool in preventing both internal and external corrosion. WW
About the Author:
Ted Huck is vice president of sales and marketing for MATCOR Inc., headquartered in Doylestown, PA, with an office in Houston. He holds a BS in Electrical Engineering and a Masters in Business Administration. He has worked with clients worldwide on cathodic protection applications in water and water treatment plants, pipelines, power plants, condominiums, parking garages, above ground storage tanks and more. Huck can be reached at [email protected] or www.matcor.com.