Amperometric vs. Colorimetric Methods for On-line Measurement of Chlorine
By Lori McPherson
Continuous on-line measurement of chlorine is critical for drinking water and many other applications. Beginning in November 2009, the EPA officially recognized amperometric measurement in addition to the previous standard of colorimetric, or the DPD method. Both methods offer accurate readings of free chlorine concentration, but the method of determination is significantly different. The user should also consider other factors such as speed of response, required system maintenance, and overall cost when choosing a system.
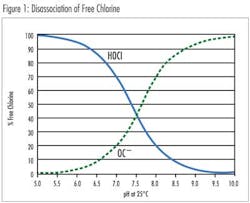
Free chlorine is the sum of chlorine gas (Cl2), hypochlorous acid (HOCl) and hypochlorite (OCl–). Above pH 4.0 all of the molecular chlorine is converted to HOCl and OCl–. Hypochlorous acid is a more potent disinfectant than hypochlorite and exists in a pH dependent equilibrium. Free chlorine also combines with naturally occurring or human-introduced nitrogen compounds in the water to form chloramines, also known as combined chlorine. Treatment operators introduce ammonia into the water to form Chloramines. Monochloramine (NH2Cl), dichloramine (NHCl2) and trichloramine (NCl3) are less effective as disinfectants but have a longer residence time than the free chlorine species. Total chlorine is the sum of free chlorine (Cl2, HOCl and OCl–) and combined chlorine (NH2Cl, NHCl2, NCl3).
Both DPD and Amperometric methods can be fine-tuned (change of sensors, reagents and/or electrolytes) to read either free chlorine or total chlorine.
DPD Colorimetric Analyzers
The long time standard for chlorine measurement is DPD colorimetric detection. This is based on N,N-Diethyl-p-Phenylenediamine (DPD) which reacts with the active free chlorine* to form a colored product that is read photometrically to determine the amount of chlorine present.
The standard on-line DPD analyzer for this uses small valves and peristaltic (tube) pumps to deliver specific amounts of process water, DPD reagent and buffer solution to the measuring cell.
Sample flow to the analyzer should be in the range of 200-500 ml/min with pressure nominally at 1.5 psi (a sample conditioning system is provided to reduce pressures from a maximum 75 psi). An advantage of this system is that the measurement is independent of temperature, pH and sample flow/pressure fluctuations. Discharge from the analyzer is sent to drain. Some consider the waste stream generated to be a major drawback as the chemicals present in the analyzer discharge may be harmful to the environment, but in general these are at very low concentrations and below Maximum Contamination Levels (MCL). The operating range for DPD reagent measurement is 0-5 ppm free (or total) chlorine, with accuracy ± 5% or .04 ppm, whichever is greater. The DPD method suffers from interferences of certain iron and manganese species.
The analyzer is shipped pre-calibrated to colorimetric standards. If necessary, the instrument can be calibrated on-site using either a standard solution (requires temporary modification of plumbing to draw a sample from the standard solution instead of the process) or can be process calibrated by adjusting the reading to match an external test. The on-site calibration using standard solutions does carry a warning for a chemical exposure hazard.
Standard maintenance of the DPD colorimetric analyzer is to replace bottles of reagent and buffer solutions on a monthly basis. Pump tubing needs to be replaced on a 3-6 month interval (shorter intervals for higher temperature (>80F) applications). All other tubing in the analyzer should be replaced annually. There are many other small fittings and parts within the analyzer that will also fail over time and require replacement. Most assemblies in the unit are quite small, and working on the unit requires instrumentation sized tools. If there is the potential for solids in the water, it is necessary to install an upstream filter as the small diameter tubing can become clogged with particulate.
Amperometric Analyzers/Controllers
The standard amperometric sensor design consists of two electrodes** (anode and cathode) that measure a change in current caused by the chemical reduction of hypochlorous acid at the cathode. The current that flows because of this reduction is proportional to the chlorine concentration. A membrane and electrolyte help to control the reaction. Flow rate and pressure must be carefully controlled for accurate measurement.
The effect of pH on the disassociation of hypochlorous acid to the hypochlorite ion is quite significant. A standard sensor design is suitable for a constant pH in the range of 6.8-8.0. Calibration of the system enables compensation for the pH of the sample. For applications with varying pH, or pH values beyond this range, an extended pH range sensor is available using a 4.0 pH electrolyte in the membrane cap. This enables conversion of the hypochlorite ion to hypochlorous acid, enabling accurate chlorine readings in solutions between pH 4.0-12.0.***
Other options for pH compensation in amperometric systems use a pH measurement and compensation in the analyzer for changes in pH.
Interferences for amperometric free chlorine measurement include ozone and chlorine dioxide. It should also be noted that amperometric measurements require temperature compensation to account for changes in membrane permeability and electrolyte conductivity. These effects are determined by a calculation in the analyzer/controller, but could lead to some error at higher temperatures.
The sensors are relatively free from interference. Manganese, iron, nitrate, and chromate that cause interference with other methods have little effect on amperometric sensors. High turbidity, oils or organics in the process water are the greatest problems for amperometric membrane fouling requiring frequent cleaning, calibration, and/or membrane cap replacement, but these are fortunately not commonly found in drinking water applications.
The amount of time to achieve an accurate reading is only 30 seconds for a standard sensor, two minutes for an extended pH range sensor. Flow to the sensor must be consistently controlled in the range of 8-26.4 gal/hr; with a maximum pressure of 1 atm (discharge of sensor must be to atmospheric pressure). Each sensor typically has a required flow cell configuration to enable accurate measurements. During initial start-up, the sensor must be conditioned in the flow stream for 12-24 hours before attempting any calibration.
Discharge from the sensor is sent to an open drain to meet the 1 atm pressure requirement. This may be considered a disadvantage because of the large volume of water going to drain, but there are no hazardous chemical considerations.
Once the system is started, it is important to maintain consistent flow to the sensor, or the system may require recalibration. In addition, loss of flow to the sensor may require the membrane to be replaced. Typical maintenance for an amperometric system is to replace the electrolyte in the membrane every 3-6 months, and replace the membrane once a year. The sensor must be re-conditioned for several hours after a membrane or electrolyte replacement. The membrane is part of a screw-on cap that is easily filled with electrolyte from a bottle.
Calibration is required after initial conditioning, and then typically about once a week by comparison to a hand-held DPD test. This calibration enables the system to compensate for changes in electrode surface, electrode fouling, electrolyte depletion, and membrane fouling. System flow and pressure must remain at a consistent level to maintain overall accuracy. Resolution of the amperometric standard range free chlorine sensor is .01 ppm. Typical system cost of an amperometric system varies, but is generally significantly less than a colorimetric system.
Conclusion
In general, colorimetric systems require less calibration, but greater overall system maintenance including replacement of reagents monthly, and service as needed for a large number of other small parts. Amperometric sensors require a larger overall flow to the sensor (and to drain), but do not have any hazardous chemical considerations and are relatively interference free. Both systems can provide an accurate measurement of chlorine if the systems are properly configured and maintained.
About the Author: Lori McPherson has been a Regional Sales Manager with Walchem for 14 years. She has a B.S. in Chemical Engineering from Purdue University, and a M.S. in Systems Engineering from Virginia Tech. Prior to Walchem she has held positions as a Process Engineer, Waste Treatment Engineer, and Analytical Product Manager specializing in Conductivity, pH and ORP Measurements. She has previously published papers focused on ORP measurement and control, and accurate conductivity measurements.
*Changing the buffer solutions enables the system to read total chlorine by the addition of Potassium Iodide which chloramines convert to Iodine. The colorimeter reads the combined total of chlorine and iodine, enabling an indication of total chlorine.
**Some manufacturers split the Anode into two components, and refer to the design as a three electrode system.
*** Other available sensors include variations in range (i.e. 0-2 ppm, 0-20 ppm, 0-200 ppm), total chlorine, chlorine dioxide, hydrogen peroxide and more.