Anion exchange resins remove perchlorate from groundwater
By Daryl Gisch
Perchlorate, even at low levels, may present a potential health threat to drinking water supplies, but anion exchange resins can offer a simple solution.
Perchlorate (ClO4-) is an anion that has been introduced to the environment over a period of more than 50 years from the production of solid rocket fuels, explosives, munitions, chemical fertilisers and a wide variety of chemical and industrial processes. The anion is very persistent and long lasting, and flows freely in ground and surface waters; thus once introduced, perchlorate, even though it is diluted, can spread to affect large bodies of water. Unlike many other pollutants, perchlorate's health effects can be triggered at very low levels (parts per billion) if consumed over a period of time.
Applying an anion exchange resin, such as a DOWEX™ resin, offers a workable solution for removing perchlorate at low concentrations from water and preventing negative health consequences. Dow Liquid Separations, a division of The Dow Chemical Company, manufactures the DOWEX resin.
Chemically, the ClO4- species is generally unreactive in water. Although ClO4- is highly oxidised, it is fairly non-labile kinetically. This means that the reduction of ClO4- to less hazardous forms like chlorate (ClO3-), chlorite (ClO2-), hypochlorite (ClO-) or chloride (Cl-) proceeds very slowly. Even common reductants like thiosulphate, sulphite and similar agents show little measurable reactivity for reducing ClO4-. Therefore, once released, perchlorate may persist for decades. Since perchlorate neither floats nor sinks in water it will freely distribute and migrate with water flows. This has resulted in low-level dispersal of perchlorate over large areas from periodic releases for more than five decades.
For many years, the common assessment of perchlorate was that since it dispersed so freely, it should be present at very low levels. This proved to be true, but, in 1992, research confirmed a direct link between perchlorate and the disruption of the thyroid gland in humans. This disruptive effect can result in a condition similar to hyperthyroidism, usually associated with iodine deficiency. There have also been numerous medical reports that have linked perchlorate to Grave's disease and cancer in adults and hormonal and neurological risks in newborns.
In 1997, a California state laboratory developed a testing method that allowed for accurate testing for perchlorate down to the four parts per billion (ppb) level. Armed with this new analytical method, perchlorate was found over very widespread areas of California and in at least 21 other states. By 2000, a number of health risk assessment models suggested exposure via drinking water could affect human health at levels of 32 ppb for adults and as low as 5 ppb for infants.
A wide range of technologies is being applied to treat and eliminate perchlorate quite successfully. In general, perchlorate is not difficult to treat for, but what can be challenging is treating the low concentrations at which perchlorate is found and handling the large volumes of water that require processing.
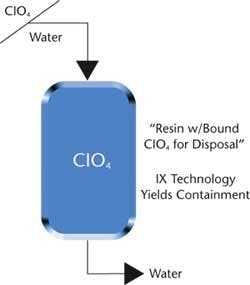
Applying an anion exchange resin offers a workable solution for removing perchlorate at low concentrations from water. Instead of regenerating the resin and creating a secondary waste stream that requires additional clean up, DOWEX anion exchange resins can be applied as a once-through processing tool. The value that a non-regenerated resin method provides is its ability to process large volumes of water while containing the perchlorate for disposal. Resin bound with perchlorate can be completely removed from a site and disposed of in a regulated landfill or sent for specialised incineration.
Of all the commercially available strong base anion resins that are capable of exchanging and removing perchlorate from a water process stream, only three currently carry the ANSI/NSF Standard 61 certification as Drinking Water System Components: DOWEX 1 (The Dow Chemical Co., USA), CalRes 2101 (Calgon Carbon Corp., USA) and Tulsion A-72 MP (Themax Limited, India).
Author's noteDaryl Gisch, Ph.D., is a developmental leader for Dow Liquid Separations, based in Midland, Michigan, USA.Federal action underwayAcceptable levels of perchlorate vary from state to state, however one US senator is trying to set a uniform federal standard. On 3 March 2003, Senator Barbara Boxer introduced a Senate Bill that would require the EPA to apply a "National Standard for Perchlorate Contamination in Drinking Water" by 1 July 2004.
Pending the legislative outcome, the problem will be better defined. Numerous water sources carry perchlorate at the 10 to 40 ppb range that warrant remediation before they are used for drinking water.