A New Method for Chromium (VI) Measurement in Drinking Water
By Wei Liu, Manali Aggrawal, Jeff Rohrer, and Richard Jack
Chromium is a naturally occurring element, widely dispersed through rocks, water, air, and soil. It has become a major concern of late as it has been detected in unhealthy concentrations within drinking water. This is not a recent issue but an ongoing challenge for health and safety agencies that need to assess safety standards for chromium levels in water and set regulations for its detection and monitoring.
Chromium (III) and chromium (VI) are the two most common forms of chromium in water. Chromium (III) exists naturally in the environment and is an essential nutrient for the maintenance of metabolic pathways in living organisms. Conversely, chromium (VI) is rare in nature, being primarily produced and introduced into the environment by industrial processes. It is known to be toxic, causing skin diseases on contact, lung cancer if inhaled, and possibly other cancers if ingested. As chromium (VI) is a strong oxidizer, it is immediately harmful to biological systems and thus warrants strict regulation.
Chromium Regulations
While safety organizations have set total chromium concentration limits in water, no current standard for chromium (VI) exists, even though several studies point to its severe health hazards — even at extremely low levels. Currently, the World Health Organization (WHO) has a set provisional guideline value of 0.05 mg/L for total chromium,1 while the United States Environmental Protection Agency (US EPA) set a Maximum Contaminant Level (MCL) at 0.1 mg/L for total chromium in 1991.2 The EPA is however still reviewing and evaluating the health assessment data and no decision for a change in the regulatory standard has been made.
Interestingly, the State of California is the only state that issued a regulation on chromium (VI) with a Public Health Goal (PHG) at 0.02 µg/L in 2011 and a MCL at 0.01 mg/L in 2013, based on research stating that exposure at these levels would cause negligible effects over a lifetime. It’s important to note that in May 2017, a court ruling invalidated California’s MCL for chromium (VI) on the grounds that the economic feasibility of compliance was not properly considered. The state is currently working on a new MCL but, regardless, inadequate standards for speciation present a challenge in meeting any low-level health goals.
Conventional Analytical Methods
Alternative methods for chromium (VI) measurement have been adapted to improve the detection limit of this specific compound.3,4 A frequently applied method for chromium (VI) detection, EPA Method 218.6,5 uses anion-exchange chromatography with post-column derivatization, providing a detection limit of 0.3-0.4 µg/L for chromium (VI). Although this post-column derivatization method is very sensitive, it requires manual preparation of the eluent and the post-column reagent, and installation of a post-column reaction coil to mix the reagent with the column effluent. These steps can be time-consuming and create additional potential for human error.
EPA Method 218.76 was subsequently developed with the intention of creating specific chromium (VI) monitoring at an even lower Method Detection Limit (MDL) ranging from 0.0044 to 0.015 µg/L. Method 218.7 was also updated to increase sample preservation and holding times, making the method much more user friendly. Compared to EPA Method 218.6, Method 218.7 has an adjusted flow rate and reaction coil size for better sensitivity to improve the detection limit.7,8 These changes are made possible by a smaller analytical column size.
A New Method Using Suppressed Conductivity Detection
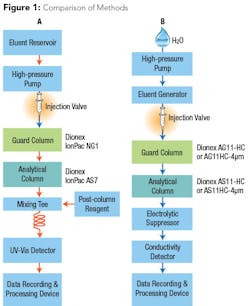
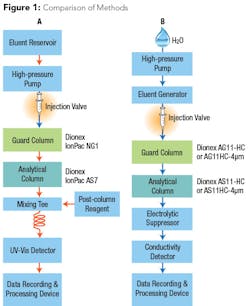
In order to eliminate post-column derivatization but maintain sufficient sensitivity to meet low-level health goals, a new method was developed that takes advantage of reagent-free ion chromatography (RFIC) and automatically generates a hydroxide eluent. Because the hydroxide eluent is suppressed to water, background noise is reduced to a much lower degree than that observed using a carbonate eluent, amplifying the analyte signal prior to entering the conductivity detector (see Fig. 1). This instrument configuration allows the method to determine 2 µg/L chromium (VI), even in high-ionic strength samples.
In this new method,9 a high-capacity column separates chromium (VI) by binding a large number of inorganic ions and organic acid anions while separating them from chromate using hydroxide eluent. Even High Ionic Water (HIW), a very challenging simulated drinking water matrix for trace anion determinations, can successfully detect chromium (VI). Figure 2 shows the detection and quantification of 1, 2, 3, 4, and 5 µg/L chromium (VI) in a HIW matrix.
The method detection limit (MDL) determined for chromium (VI) is 1 µg/L with a minimum quantification limit of 3 µg/L, sufficient for measuring the chromium (VI) concentration at very low contaminant limits.
The new approach provides several advantages over previous methods. Automating the hydroxide eluent generation from water saves time and reduces errors caused by manual eluent preparation, as is used in the EPA Methods 218.6 and 218.7. Employing high-capacity analytical columns eliminates any potentially problematic matrix effects from high ionic strength waters. Using an electrolytic suppressor reduces the detection background while amplifying the signal for direct conductivity measurement of chromium (VI) without using post-column derivatization. These updates create a highly specific method that allows chromium (VI) separation and measurement while reducing labor-intensive steps and maintaining low detection levels.
Method Applications
The new suppressed conductivity method is less sensitive than EPA Method 218.6 (0.3 or 0.4 µg/L) and 218.7 (0.0044 to 0.015 µg/L), providing a higher MDL of 1 µg/L. However, the new method omits the post-column derivatization step and thus saves time and reduces errors. With RFIC systems and high-capacity columns in place, the new method is simpler and easier to operate, achieving limits for monitoring and analysis of chromium (VI) in drinking water when the minimal reporting level is higher than 3 µg/L. The approach also enables the quick assessment of chromium (VI) in drinking water and environmental water when the level of chromium (VI) is unknown, and easy adaptation for the detection of chromium (VI) in soil and solid waste after proper sample preparation.
Future Considerations
Although the EPA is still assessing health effects data, research shows that two thirds of the U.S. population is exposed to a dangerous level of chromium (VI) in drinking water. Regardless of whether national regulations for chromium (VI) will be developed in the near future, chromium (VI) testing will most likely become more common both nationwide and worldwide. Therefore, the quick, easy and accurate detection of chromium (VI) by suppressed conductivity could become the basis for robust detection while providing a simpler and faster analysis to meet possible new regulatory standards. WW
About the Authors: Wei Liu, Ph.D., is a senior market development manager with a focus on trace elemental analysis applications in environmental and industrial markets in the Chromatography and Mass Spectrometry group at Thermo Fisher Scientific Inc. Manali Aggrawal, Ph.D., is a senior applications chemist at Thermo Fisher Scientific, with extensive experience in analytical instrumentation. Jeff Rohrer, Ph.D., is the director of applications development for Dionex Products at Thermo Fisher Scientific. Richard Jack, Ph.D., is the senior director of vertical marketing for the environmental and industrial markets for the Chromatography and Mass Spectrometry group at Thermo Fisher Scientific.
References
1. “Chromium in drinking water,” World Health Organization, 2003.
2. “Chromium in drinking water,” U. S. EPA website, www.epa.gov, accessed Nov. 2017.
3. “Determination of Hexavalent Chromium in Drinking Water Using Ion Chromatography,” Thermo Fisher Scientific Application Update AU144, 2003.
4. “Sensitive Determination of Hexavalent Chromium in Drinking Water,” Thermo Fisher Scientific Application Update AU179, 2011.
5. “Method 218.6: Determination of Dissolved Hexavalent Chromium in Drinking Water, Groundwater, and Industrial Wastewater Effluents by Ion Chromatography, Rev. 3.3,” Office of Research and Development, U.S. EPA, 1994.
6. “Method 218.7: Determination of Hexavalent Chromium in Drinking Water by Ion Chromatography with Post-Column Derivatization and UV-Visible Spectroscopic Detection,” Office of Water, U.S. EPA, 2011.
7. Jack, Richard F., Jeffrey S. Rohrer and Lipika Basumallick. “The changes from EPA method 218.6 to 218.7,” The Column, Chromatographyonline.com, 2012.
8. Eaton, Andy. “Hexavalent Chromium and Total Chromium in Natural Waters – Can We Ensure Accurate Measurements at Sub-ppb Levels?” Presented at the National Environmental Monitoring Conference, 2012.
9. “Determination of Hexavalent Chromium Cr(VI) in Drinking Water by Suppressed Conductivity Detection,” Thermo Fisher Scientific Application Note AN1116, 2016.