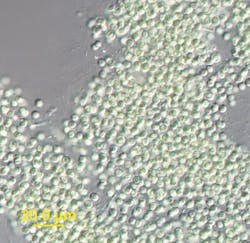
The Blue-Green Monster: How Harmful Algal Blooms Are Increasing Costs, Risks for WTPs
By Sarah Fister Gale
In August 2014, the entire city of Toledo, Ohio, lost access to drinking water for three full days due to a sudden deluge of microcystin in the source water. Microcystin is a cyanotoxin found in some cyanobacteria -- the blue-green algae blooms that grow on surface water in warm weather. These toxins can add a foul odor and taste to water and potentially lead to nausea, rashes, dizziness, and even liver and kidney damage.
The incident in Toledo was caused by a combination of bad luck, high winds and possibly insufficient monitoring, explained Jeffrey Reutter, director of the Ohio Sea Grant College Program at the Center for Lake Erie Area Research and the Great Lakes Aquatic Ecosystem Research Consortium. "The bloom as a whole on Lake Erie was not very big last summer," he said. But during the day, when algae blooms float, strong winds blew a bloom against the water intake pipe. At night, when blooms tend to sink, the algae was sucked into the system. "As a result, the toxin concentration was really high, and the plant couldn't remediate enough of it to achieve necessary safe drinking water levels," he explained.
It was a cyanotoxin "perfect storm" that, admittedly, rarely occurs. In most cases, water utilities can monitor for and manage the presence of cyanotoxins in drinking water sources to prevent such crises, but the situation in Toledo highlights the real risk they face every summer as the presence of these blooms increases. "Cyanotoxins are a growing concern, and they are not going to go away," said Tom Curtis of the American Water Works Association.
Out of Their Control
Cyanotoxins have become an ever-present adversary for cities that draw their drinking water from surface reservoirs. According to the Environmental Protection Agency (EPA), drinking water sources in all 50 states are affected by these potentially harmful algae blooms, which occur in slow-moving water with high levels of phosphorus and nitrogen. While some of these nutrients enter waterways through poorly managed septic systems and urban runoff, the largest source of nutrient loading comes from farming operations with poor land management practices and extreme overuse of fertilizers and manure. Curtis noted that some farms use four to five times more fertilizer than the soil can absorb, and the rest just runs into the waterways. Unfortunately, there are few laws to prevent such runoff, he said. "Agricultural practices are largely outside of the jurisdiction of the Clean Water Act."
There are also no current regulations from the EPA for safe levels of cyanotoxins or microcystin in drinking water, although the Agency is paying close attention to this issue, said Eric Burneson, director of the standards and risk management division for EPA. The Agency has identified cyanotoxins on its Candidate Contaminant List and recently published a fact sheet on sampling and treatment methodologies. However, there are currently no enforceable regulations. "Getting on the Candidate Contaminant List is the first step to regulations, but it is very prospective at this point," he said.
In the meantime, water utilities must manage the related mess that these algae blooms create -- and rate payers are left to pick up the cost.
What's Monitored Can Be Managed
Burneson explained that since the growth and risk of cyanotoxins in surface water is unavoidable, treatment plant operators should develop a contingency plan of action should these toxins be detected. Most utilities take a two-pronged approach: they monitor regularly for the presence of cyanotoxins in the water, and then, when they occur, they implement a multi-tiered elimination process to ensure water quality is maintained.
EPA suggests that each plant create a cyanotoxin monitoring plan that outlines when and where sampling will take place, how often, and what screening tools and processes will be used to ensure consistent tracking of these contaminants. The Agency recommends using Enzyme-Linked Immunosorbent Assay (ELISA) test kits for initial sampling, as they are inexpensive and relatively easy to use. However, if the presence of cyanotoxins is detected, treatment plant operators may need to invest in more complex mass spectrometer equipment or work with a local lab to precisely identify the level and species of toxins in the water, Burneson said.
Such proactive monitoring ensures that drinking water quality and safety can be maintained without overwhelming a utility's treatment budget, explained Richard Stumpf, oceanographer at the National Oceanic and Atmospheric Administration (NOAA) in Silver Springs, Md. "If you aren't prepared to deal with cyanotoxins, it can be an expensive problem to address," he said. Because these blooms rise to the surface during the day and sink at night, treatment plant problems usually occur in the middle of the night. That means calling in extra people and paying them overtime, and finding suppliers who can deliver activated charcoal and other treatment materials right away. "It's much cheaper to plan ahead," he advised.
To help local utilities stay ahead of these toxins, NOAA conducts satellite monitoring of Lake Erie and offers daily forecasts on the size and location of algae blooms, which gives plant operators a heads up to what's coming. Reutter's team at the Ohio Sea Grant College Program offers workshops in the summer that cover water treatment plant management strategies for harmful algal blooms. "They teach plant operators how to know when they need to take action and what actions to take," Reutter said.
The Alliance Method
Dean Reynolds, superintendent of the Water Utility Council in Alliance, Ohio, has taken his own proactive approach to managing and mitigating cyanotoxins. He conducts weekly sampling of his utility's two reservoirs to both identify the species of algae in the water and count the number present. "We always want to know when there is an algae bloom and what's blooming," he said. The Ohio EPA provided his facility with a grant to buy equipment needed to run the tests.
When the bacteria is present, which it inevitably is in the summertime, he uses an innovative multi-barrier approach to eliminate it.
One of the challenges related to cyanobacteria treatment options is that many toxins exist inside the bacteria cell. That means when the cell dies or its membrane ruptures, the toxins are released into the water, potentially worsening the problem. "Ideally, you want to avoid that," Reynolds said. However, it impacts the kinds of treatment options that are used. For example, a treatment plant may use chlorine dioxide as a preoxidant to control taste and odor, but this step can destroy the cell membrane. Similarly, copper sulfate and ozone at the intake removes the algal bloom but is not recommended because of the risk of lysing algal cells. "If you can coagulate the particles before destroying the cells, you can take a lot of the contaminants out of the process," he explained.
Reynold's plant uses powder activated carbon (PAC) made from wood as a first barrier. Before selecting the wood-based PAC, his team compared the efficacy of several materials, including coal, lignite and coconuts, and found that wood removed twice as much material as other carbons. "The wood is more effective for us, and we can use it at a lower application rate," he said. This approach saves the utility money and speeds up the elimination process. Reynolds noted, though, that every plant is different. "You may need to spend a few hundred dollars to test which carbon is most efficient for your needs, but it can save you a lot of money in the end."
EPA also suggests potassium permanganate, which can be effective in removing cells with no release of toxin in combination with PAC.
Reynold's next barrier is a filter capped with granular activated carbon (GAC). "The GAC becomes biologically active in the summer, which means the microbes remove chemicals even better than removal by adsorption alone," he said.
Last year, his plant installed two new ultraviolet (UV) light reactors for advanced oxidation as a final barrier step. The process involves injecting hydrogen peroxide upstream of the reactors; the UV rays then split the hydrogen peroxide into hydroxyl radicals, which attack the organic chemicals in the water. "UV advanced oxidation is cutting-edge technology for drinking water treatment," Reynolds said. When he started looking into the solution in 2009, only four plants in the world were using it to remove organic chemicals from drinking water. He estimated that less than 30 plants in the U.S. currently use this technology.
Reynolds multi-barrier approach enables his team to stay ahead of the cyanotoxins that might develop in the water. In the meantime, he's encouraging local farmers and nearby communities to improve their land management practices and update aging septic systems to lower the nutrient loading in his reservoirs. "It's hard because we have no control over what they do, but I keep pushing," he said.
And that's likely the best that a utility can do. "The lack of regulations leaves drinking water utilities and rate payers as the victims of a pollution problem that someone else is causing," Curtis said. He is hopeful that continuing education about effective land management practices, coupled with tighter regulations in the future, could stem the occurrence of these harmful -- and expensive -- algae blooms. "Prevention makes a lot more sense than treatment," he said.
About the Author: Sarah Fister Gale is a freelance journalist based in Chicago, Ill. Over the last 15 years, she has researched and written dozens of articles on water management trends, wastewater treatment systems and the impact of water scarcity on businesses and municipalities around the world.
A Word About DisinfectionIn the process of drinking water treatment, disinfectants are utilized to satisfy two objectives: The first is to kill or inactivate disease-causing organisms, and the second is to leave a residual amount of disinfectant for use in the distribution system. Chlorine is commonly used as a disinfectant in drinking water treatment and may be added as elemental chlorine, sodium hypochlorite or calcium hypochlorite. Regardless of the form that is added, the pH of drinking water plays a vital role in its efficacy. Hypochlorous acid (HOCl) is the effective disinfecting component of chlorine and is predominant at values lower than pH 7; the hypochlorite ion (OCl-) component is predominant at values higher than pH 8. The pH of drinking water should be kept at a neutral pH during and after disinfection to ensure that there is a sufficient amount of HOCl present. The pH can be tested using an electrode and meter or a digital controller.
More WaterWorld Current Issue Articles
More WaterWorld Archives Issue Articles