On-site NaClO generation avoids transport hazards
By: Steve Mickey
On-site electrolytic generation of sodium hypochlorite is gaining popularity for use in seawater desalination plants due to the hazards of gaseous chlorine and commercial grade sodium hypochlorite (NaClO).
Natural sources of salt water contain copious amounts of flora and fauna that, if left unchecked, grow inside the process piping and equipment and eventually render the equipment useless. These organisms (or bugs) need to be disinfected or removed from raw water by chemical treatment or filtration. Typically, chlorine is used to treat the raw salt water because it is less expensive than filtration. This chemical can be obtained commercially in the form of gas or concentrated NaClO solution or generated on-site electrolytically.
Electrolytic generation of NaClO has been used for decades, but the low capital cost associated with the use of gaseous chlorine and commercial grade NaClO in seawater desalination facilities limited its user base. Increasing awareness of environmental and community safety issues in the past few years has made on-site NaClO generation now a more attractive disinfection method. Upfront capital costs for the required equipment is relatively high compared to gaseous chlorine, but operational costs are exceptionally low. NaClO generation systems require only power and salt water to operate. The typical payback period for a system using seawater is approximately five years.
Coastal desalination facilities often opt for hypochlorite generation systems given the minimal raw materials required on-site.
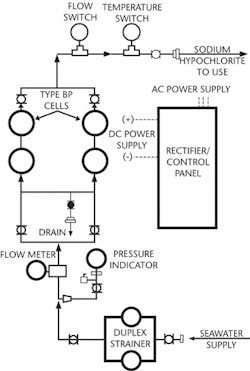
The operation of a NaClO generator is fairly simple. Salt water is pumped through a specially designed cell that is charged with DC electricity. An electrochemical reaction takes place within the cell breaking apart the salt and water molecules, which then recombine to create the NaClO solution. This bleach solution, which is typically generated at concentrations of 1,000 to 2,000 milligrams per litre (mg/l), is then injected back into the saltwater source at the intake structure at rates typically around two mg/l. When the NaClO contacts the "bugs," it releases its oxygen and turns back into sodium chloride (salt). After the treated water passes through the process the discharge water contains a residual chlorine concentration often as low as 0.1 mg/l making it suitable for releasing into the ocean.
PEPCON Systems provided a ChlorMaster hypochlorite generation system that supplies sodium hypochlorite for two different applications to a desalination plant expansion project in the United Arab Emirates. The facility uses approximately 200 million gallons per day (mgd) of seawater to produce more than 50 mgd of potable water. The system is comprised of three units using the PEPCON bipolar type electrolytic cells. Two of the three units are each designed to provide up to 100 kg/hr NaClO for treating seawater before it is used in the desalination process. The third unit is sized to generate up to 36 kg/hr NaClO for drinking water disinfection. Togeth-er, the ChlorMaster units provide all of the NaClO used at the site. This system runs automatically and produces hypochlorite based on the quantity required to maintain a preset chlorine residual.
Author's note
Steve Mickey is the applications engineer of PEPCON, based in Cedar City, Utah, USA.