Guest Commentary: Diamond Electrodes Enable Electrochemical Advanced Oxidation Processes for Industrial Wastewater Treatment
Rather than transporting wastewater to treatment plants, effective treatment at the generation point of the effluent minimizes the risk of contamination and also makes recycling industrial wastewater viable, dramatically improving water use efficiency. Various treatments that originated from sewage and wastewater treatment have been used to treat industrial wastewater. All of them have inherent problems that make them less than ideal processes for removal of recalcitrant compounds such as phenolic, and biocides such as ammonia; often, even in large central plants, these types of wastestreams are challenging to treat.
Advanced oxidation processes (AOPs) have the ability to mineralize recalcitrant organic and inorganic contaminants in toxic and biocidal wastewater streams, via the creation of hydroxyl radicals that have the oxidation power to treat these types of effluent streams. AOPs have the advantage of being suitable for small- to medium-scale treatment systems that are straightforward to operate at the point of need.
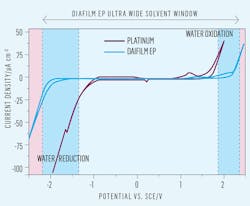
enabling high oxidation efficiencies for EAOPs; its water oxidation window is
at a much higher potential than metallic electrodes such as platinum.
Most common AOPs use a strong oxidizing reagent such as hydrogen peroxide or ozone in combination with ultraviolet light activation to generate hydroxyl radicals. Electrochemical AOPs (EAOPs) generate the hydroxyl radical directly at the surface of electrodes placed in the effluent stream. EAOPs are attractive as they are simpler to operate and do not require purchase, storage, and handling of strong oxidizing reactive reagents, which are hazardous in their own right. However, the first generations of EAOPs have been largely ineffective, due to the lack of a commercially feasible electrode that could generate sufficient concentration of hydroxyl radicals efficiently, while withstanding the harsh environment of electrochemical oxidation. Electrochemical hydroxyl radical generation requires the application of such extremely high electrode potentials that most electrically conductive materials themselves oxidize, including titanium-based dimensionally stable anodes and lead dioxide versions.
Diamond is a semiconductor that can be heavily doped with boron, giving it metal-like electrical conductivity, while retaining its renowned robust properties. Electrochemical oxidation electrodes using boron-doped diamond (BDD) have been researched for more than 20 years. BDD’s inert surface suppresses the electrolysis of water, enabling the required electrode potentials to be applied to generate hydroxyl radicals and thereby finally making EAOPs that were effective. However, these second generation EAOPs used thin film BDD coatings that still did not have the lifetime to make industrial scale-up viable.
These challenges, which have prevented widespread industrial adoption of EAOP technology for over 10 years, have recently been overcome by the use of free-standing, solid polycrystalline, BDD electrodes.
With no substrate, solid BDD electrodes have an extremely wide solvent window and their inherent stability enables very high current density operation combined with an electrode lifetime measured in years.
The other reason EAOPs have been investigated less extensively is that they are perceived to consume high energy and have a relatively higher cost than chemical dosing. One of the reasons for this is that traditional metal-based electrodes only had limited effectiveness in generating advanced oxidation conditions, since too much of the electrical power was wasted in the electrolysis of water and did not go into the oxidizing of the pollutants. However, the amount of energy used per pound of chemical oxygen demand (COD) or ammonia can be reduced with BDD electrodes since its wider solvent window reduces the power lost due to spurious oxygen and hydrogen evolution. Finally, in an electrochemical process, the work done is proportional to the total charge flowing through the cell. Depending on the process, solid BDD electrodes can operate in a range greater than 20,000 ampere per square meter, making compact electrochemical cell systems with BDD electrodes a cost effective solution due to the amount of oxidation work that can be achieved.
EAOPs with BDD electrodes are an environmentally friendly means of treating wastewater contaminated with toxic and persistent herbicides, pesticides, chlorophenols, nitrophenols, polychlorinated biphenyls, pharmaceuticals, etc. Dissolved recalcitrant species are treated either by full oxidation to CO2, or by breaking them down into compounds that are more readily biodegradable in conventional treatment processes. EAOPs have been found effective for a wide range of complex wastestreams, including high COD- and ammonia-containing streams such as landfill leachates and for treating process waters in textile, semiconductor, and pharmaceutical manufacturing, to name but a few.
Now that the limiting factors of this technology have been mitigated, new wastewater plants are being designed and installed all over the world. BDD EAOPs are a sustainable technology that is preferred over chemical or ultraviolet AOPs for treatment of recalcitrant industrial wastewater streams for many environmentally conscious plant managers. The next step will be to use this technology to recycle process water for a more sustainable and efficient use of water in our industrial processes.