Improved Chloramination Stabilizes Distribution System
Water treatment operators at the City of Longview, TX, Lake O’ the Pines Water Treatment Plant are old hands at chloramination - yet they have found a new, practical means to finesse the ratio of injected chlorine and ammonia during disinfection. Since July 2004, the Longview operators have been using a utilitarian water quality test specific for free ammonia to tightly control chemical dosing. As a result, they are documenting a more stable monochloramine residual and less free ammonia in the distribution system - conditions that keep nitrification at bay. And, as a bonus, they have significantly reduced chemical consumption.
Ammonia Control
Disinfection with chloramines is the most widely applied alternative to chlorination in the United States. Water utilities using chloramination benefit from a reduced likelihood of harmful disinfection by-products (DBPs) formation in the distribution system. Further, the monochloramine (NH²Cl) residual, while less powerful as a disinfectant than free chlorine, is more persistent in the water network. Finally, although ammonia feed is necessary, less chlorine is required to form NH²Cl, typically making the process an economical chlorination alternative.
The production of chloramines for water disinfection is not without its own set of water quality issues. Operators should avoid adding excess chlorine or ammonia that wastes money. Further, free (unreacted) ammonia released to the distribution system can lead to a train wreck of consequences, including microbiological re-growth that induces pH change, corrosion, and monochloramine decay that, in turn, fuels further biofilm growth (nitrification) and heterotrophic plate count (HPC). Costly nitrification remediation steps such as shocking with free chlorine, and the related taste, odor, and customer notification issues related to flushing, are significant liabilities that concern water treatment facilities considering chloramination disinfection.
Dosing the correct ratio of chlorine to ammonia (as nitrogen) to maximize monochloramine concentration and minimize free ammonia is, theoretically, a straightforward process. In reality, the process is complicated when source water exhibits seasonal changes that lead to varying pH, temperature, ammonia levels, and chlorine demand properties that all impact the theoretical chlorine-to-nitrogen ratio. Reliable, real-time monitoring of key water quality parameters during chloramination allows operators to maintain the proper chlorine-to-nitrogen ratio that minimizes free ammonia, the food source for microorganisms involved in distribution system nitrification. (See sidebar for dynamics of the Chlorination Breakpoint Curve.)
Monitoring
The Lake O’ The Pines Plant, one of three water treatment facilities of the City of Longview Water Purification Division, is designed for 10 mgd capacity and receives water from its namesake lake. Treatment is typical of many surface-source plants and consists of primary ozone disinfection; high-rate solids contact clarification; chlorine addition; filtration through four, mixed-media filters; then pH adjustment and ammonia feed.
Chloramination has been applied at the city’s water treatment plants for 20 years, so ammonia monitoring is a familiar task for Senior Utility Plant Operator Trena Fischer, a City of Longview water operator. For more than 20 years, she has worked with chlorine/ammonia-nitrogen ratios at all three facilities and within the water distribution network.
With decades of experience, Fischer still welcomes ways to better manage chloramination chemical ratios. Recently she began using a new, on-site test for free ammonia that applies an indophenol method instead of the traditional Nessler ammonia method that is notorious for susceptibility to interferences, including amines such as monochloramine. Nesslerization further carries the onus of having a mercury component.
With this new colorimetric test, from Hach Company, Fischer and fellow Longview operators scrutinize dosing samples for ammonia-nitrogen levels as low as 0.02 mg/l in a matter of minutes on a laboratory spectrophotometer. They also monitor samples from distribution system points with a handheld, direct-reading Hach colorimeter. Fischer said the new Hach test is simpler and faster, and provides a more stable reading, than the Nessler ammonia test she had used for years, making it a more precise treatment control tool.
“An operator wants to see a good, stable value. I like to hit a button and get a reading, period,” she said.
The utility of the free ammonia test has helped provide more insight to the chloramination process for veterans at the City of Longview water treatment plants. With more reliable monitoring, they have been able to reduce chlorine doses nearly in half, from approximately 4.8 mg/l to 2.7 mg/l, an accomplishment that cuts the chlorine/ammonia-nitrogen weight ratio ahead of the filters from 9.0:1.0 to 5.0:1.0. Treatment target is a monochloramine concentration of 2.2 mg/l leaving the plant.
Fischer anticipates, with rising seasonal temperatures, that doses will be increased to achieve a monochloramine level of about 2.5 mg/l. Samples will be tested daily during this time, and she is confident the improved dosing ratio will be maintained.
“We’ve got it pretty much nailed,” Fischer said. “Outgoing free ammonia ranges from 0.05 to 0.12 mg/l but typically posts at about 0.1 mg/l, compared to about 0.36 mg/l prior to our new chemical dosing regimen. We’ve decreased ammonia consumption nearly 40%.”
Test Supports Network Surveillance
Operators collect 82 samples a month throughout the Longview distribution network for permit-required bacterial counts and total chlorine residual. Because ammonia values can increase due to natural auto-decomposition of chloramines, the operators rely on the dedicated handheld Hach colorimeter to keep an eye on both monochloramine and free ammonia levels in samples from those points. Their monthly survey gives them the opportunity to identify the early warning signs of nitrification.
“Generally, what leaves the plant is what we find in distribution,” Fischer said. “We see about 1.8 mg/l monochloramine at our most distant sampling point approximately five miles from the plant. We can just about bank on it. With a better dosing regimen, we can count on a more stable chlorine residual.”
Lake O’ The Pines Water Quality Supervisor Selina Tabor concurred. “With less free ammonia in the system, we’re limiting microbial growth and nitrification conditions - and calls from customers.”
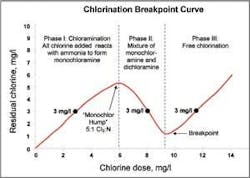
The goal of the chloramination process is to combine chlorine and ammonia in water in a manner that maximizes the concentration of monochloramine (NH²Cl) and limits free chlorine and free, or unreacted, ammonia. Free ammonia exists as dissolved ammonia gas (NH3) or as the ammonium ion (NH4+), depending on pH and temperature.To meet this goal, operators should keep the process in Phase I, where all added chlorine and ammonia react to form monochloramine. At the end of this phase of reaction - the “Monochloramine Hump” - all ammonia has reacted and no free ammonia is present.
If chlorine dosing continues, the process moves into Phase II, the chlorine/ammonia-nitrogen ratio exceeds 5:1 (w/w), and dichloramine (NHCl²) will form. Dichloramine can impart taste and odor to the water.
As the combination of monochloramine and dichloramine, or combined residual chlorine, increases with increasing chlorine dosage, measured residual chlorine actually decreases until it approaches zero - the chlorination process point referred to as the breakpoint. Chlorine added in excess of the breakpoint results in the presence of free residual chlorine.
Operators monitoring total residual chlorine alone during dosing would not be able to differentiate whether a measurement of 3 mg/l reflects Phase I, Phase II, or Phase III of the breakpoint curve. With reliable water quality tests specific for monochloramine and free ammonia, operators are assured the process is in Phase I of the breakpoint curve and that they are minimizing excess chemical feed and reducing nitrification potential.