Amperometric Probes or DPD Analyzers: Which Is Best For On-Line Chlorine Monitoring?
By Jim Huntley and Dr. Vadim Malkov
Process instruments for on-line chlorine monitoring have become essential tools for many. The Surface Water Treatment Rule suggests continuous monitoring of residual chlorine on distributed water for systems serving more than 3,300 people. Continuous on-line chlorine monitoring is used extensively at the point of distribution, in reverse osmosis systems to preserve membranes, and in wastewater treatment to ensure regulatory compliance.
The two most common methods for on-line chlorine analysis are amperometric and colorimetric detection. DPD colorimetric detection is a method based on N,N-Diethyl-p-Phenylenediamine (DPD) reaction with active halogens. This reaction is a standard analytical approach for analysis of residual chlorine and other chlorine oxidants and is based on the formation of colored products with DPD.
Amperometry is an electrochemical technique that measures the change in current resulting from chemical reactions taking place as a function of the analyte concentration. A typical amperometric sensor consists of two dissimilar electrodes – an anode and a cathode (i.e. silver/platinum or copper/gold). Below is a general schematic of the reduction-oxidation reaction taking place in the amperometric system:
Cathode (working electrode):
- HOCl + H+ + 2ë -> Cl- + H2O
(reduction of hypochlorous acid)
Anode (reference electrode):
- Cl- + Me -> MeCl + ë
(oxidation of chloride ions)
The anode may be split into two parts – a reference and an auxiliary (or counter) electrode making the measurement more stable. Such systems are called three-electrode sensors. Typically electrodes are covered with a membrane, providing for better selectivity of the analysis. Additionally, a small electrical voltage is applied across the electrodes. In the case of no membrane, the system is called bare-electrode amperometric and in the case of no applied voltage, the system is called galvanic. From a technical standpoint, many electrochemical methods fall under the amperometric measurement category, including bare-electrode and galvanic systems, which are sometimes wrongly referred to as polarographic.
It is important to note that amperometric sensors do not use the same methodology as the laboratory amperometric titration apparatus. Additionally, it should be considered that the DPD methodology and amperometric titration are standard measurement methods that provide adequate accuracy throughout the entire measurement range. In contrast, on-line amperometric sensors are designed for process control and, therefore, provide adequate accuracy only around the calibrated set-point (normally within ± 1 ppm or ~20% of the set point).
Limitations
Currently, no “ideal” method exists for quantifying chlorine and chloramines in water. All common methods of chlorine analysis display some lack of specificity and are not adequately selective to be completely free of interferences. However, most of the limitations associated with the traditional DPD chemistry (e.g., calibration linearity, reagent stability, reaction product stability, etc.) have been addressed sufficiently through procedures and reagent formulation advanced by Hach since it introduced its first chlorine test kit based on the DPD chemistry in 1973.
Several interferences have been identified that can present limitations when amperometric sensors are used for continuous on-line process measurements. Some of the more noted variables providing interference are based on sample and sampling environments with changing chlorine concentration, pH, temperature, sample flow, and pressure; and some are application-based involving ease of use, sensor fouling, interferences and calibration. In contrast, the EPA-approved DPD colorimetric method (SM 4500G) is independent of temperature, pH, and sample flow/pressure fluctuations.
Both chemical (DPD) and amperometric methods suffer from interferences due to presence of some specific compounds. For example, there is well known sensitivity of DPD analysis to the presence of certain iron and manganese species in water. Chemically, the amperometric method is free of this interference, however amperometric sensors are more prone to fouling with the presence of iron or manganese in the sample (as well as in the presence of high turbidity), and this will result in increased cleaning and calibration frequency.
Effect of pH on Amperometric Measurements
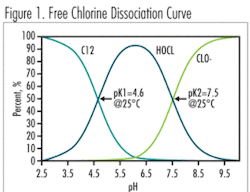
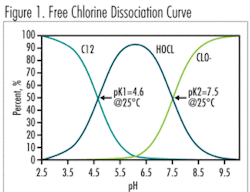
In free chlorine applications, a pH of 5.0 to 7.0 is the ideal operation range for an amperometric sensor, due to the high percentage of hypochlorous acid (HOCl) (>80%) in the sample and the steepness of the Free Chlorine Dissociation Curve (Fig. 1) in this range. The pH can move within this range and the chlorine concentration can drift without significantly diminishing the accuracy of the instrument. This pH range, however, is not naturally present in drinking water facilities.
A pH of 7.0 to 8.0 is typically the normal operating range for most drinking water facilities. The HOCl concentration is much lower versus the OCl- (hypochlorite ion) in this range. Amperometric free chlorine sensors directly measure only HOCl, not OCl- or Cl2, so any change in pH within this range will substantially affect the accuracy of the on-line unit. When pH influence is mathematically compensated, the instrument may read more consistently, however there always exists a possibility of readings drift. In contrast, the DPD method is equally sensitive to all present species, plus, it is pH independent because the sample is buffered during on-line measurement and pH of the reaction is under control.
At a pH 8.0 or greater (often the operating range for facilities experiencing problems with DBPs), the HOCl part of free chlorine concentration is very low (<20%), therefore accuracy of the amperometric probe suffers significantly with any slight changes in pH.
pH Compensation
Currently, a patented technology allows for conversion of hypochlorite ions into HOCl molecules inside the sensor cap by highly buffered electrolyte (internal pH compensation). It has been demonstrated in the field that pH compensation greatly improves the potential accuracy of on-line amperometric sensors. Where this methodology falls short is the complexity of sample matrix that additionally includes alkalinity, acidity, and hardness that cannot be compensated for with calculations in the existing technology. Having a changing and complex water matrix will lead to poor accuracy and more frequent change of the electrolyte (sensor maintenance).
In external pH compensation applications, a buffer from an external reservoir is added to the sample to adjust and control its pH. The buffer may be as simple as vinegar or as complex as necessary to provide additional benefits. Although this approach provides improved accuracy, the on-line instrumentation often loses its “reagentless” appeal due to additional ongoing expenses and waste stream containing chemicals (buffer).
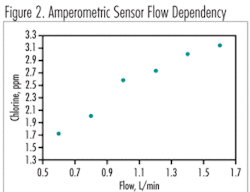
Flow/Pressure
Design of the flow cell is a critical component to the overall performance of amperometric sensors. Due to the flow and pressure sensitivity of these sensors, a flow cell design that does not adequately compensate for these changes will allow for continued poor accuracy. In some cases, amperometric sensors have been mounted in-pipe with no flow cell to condition the sample and it has been reported that the sensor’s accuracy is completely lost if the sample pressure changes more than ± 5 psi. In any membrane-based measurement system, varying sample pressure will change the thickness of the micron-sized electrolyte layer between the membrane and electrode surface, which leads to erratic responses.
There are other aspects of the flow cell that are critical in addition to flow and pressure concerns. If the on-line sensor loses sample and the membrane dries out, for example, or if the minimum flow requirement for the unit is not continuously met, calibration will be lost and then sensor re-conditioning in the sample stream followed by re-calibration is required.
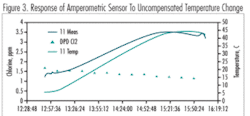
Temperature Effect/Calibration Requirements
Amperometric sensors are always sensitive to temperature changes (Figure 3). Two areas affected by temperature are the membrane permeability rate and the pH compensation, which is always done by calculation. No mathematical algorithm can accurately reflect all changes in the water matrix, and eventually the response of chlorine to those changes.
In addition, any essential changes to the water sample matrix will require recalibration of the amperometric sensor. When the water characteristics are constantly changing, this will often require weekly and sometimes daily calibration of the instrument to retain overall accuracy. In contrast, DPD technology does not require calibration due to the established proportionality between chlorine concentration and light absorbance.
Conclusion
Prior to choosing an on-line chlorine analyzer, the application should be evaluated to define what technology will be most suitable – DPD or amperometric. It is perceived that amperometric sensors designed for process control may work well in applications where chlorine concentration, sample flow, pressure, temperature and pH are stable. However, this is a perception only.
In view of the relative instability of chlorine and chloramines in aqueous solutions, especially in many process applications, as well as associated dynamic water conditions of these processes, on-line chlorine measurement using DPD is preferable for most applications. WW
About the Authors:
Jim Huntley is Global Product Manager and Dr. Vadim Malkov is Product Applications Manager, Process Instruments Business Unit, at Hach Co., Loveland, CO. Hach offers a wide range of on-line, laboratory, and portable chlorine measurement solutions, including colorimetric DPD analyzers and chlorine amperometric titrators and sensors.
Published in WaterWorld, January 2008