Enhancing RO Recovery using Intermediate Concentrate Chemical Stabilization
By Charlie He
Nearly all available water resources with good quality are fully used in central Arizona and communities are exploring the use of other water resources, such as brackish surface and groundwater. However, most of the two-stage or three-stage brackish RO systems operate at approximately 85 percent recovery. With 15 percent water loss and high energy demand, implementation of such treatment is a challenge for the state.
In a desert environment, concentrate management can no longer be simply thought of as a disposal issue, but must be recognized as an integral part of any long-term supply management strategy.
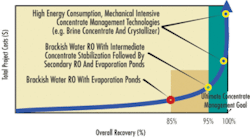
Most available concentrate management technologies can be classified into two categories. One is the low-energy-consumption, non-mechanical-intensive technologies, such as solar / evaporation ponds, chemical stabilization concentrate volume reduction, or beneficial reuse of concentrate as a resource (e.g., halophyte pond and wetlands, blending with reclaimed water for habitat restoration). The second category is the high-energy-consumption, mechanical-intensive technologies, such as thermal processes like a brine concentrator or crystallizer.
New concentrate management technologies are under development. However, all processes have certain limitations. There is no silver bullet that will meet all concentrate management needs. Therefore, an effective concentrate management strategy often involves more than one technology and is implemented in multiple steps before 100 percent recovery or “zero liquid discharge” (ZLD) can be achieved.
Recent research has shown that using a low-energy, intermediate concentrate chemical stabilization (ICCS) step followed by a secondary RO system to maximize system recovery and minimize the volume of the concentrate provides benefits in both the short and long term. Such technologies will allow desert communities to recover more good quality water from precious water resources. Even though it cannot achieve ZLD in one step, it approaches the goal at a reduced cost by reducing the volume of the concentrate.
Scaling Constituents
Typically, RO system recovery is limited by the concentration of sparingly soluble contaminants present in the feed water and the degree to which they are concentrated to levels exceeding saturation in the concentrate. As water flows through the feed side of the RO membrane, ion concentrations increase while low total dissolved solids (TDS) permeate is produced. In the final stage of the RO system, concentrations of the sparingly soluble constituents (silica, barium, calcium, magnesium, etc.) may exceed their solubility limits and form scale on the membrane surface. A manageable level of scale formation can be controlled through the use of pH adjustment, antiscalant chemicals, and RO cleaning. But if not controlled properly, scaling will impact the permeate water quality and may even cause irreversible membrane damage.
Like all bad actors, the scale-forming constituents must be identified, removed, and / or controlled. In the Southwest region, the prevalent membrane scaling constituents are often silica, barium, calcium, magnesium, and / or strontium.
Literature findings and modeling suggest two main strategies to control or remove the scale-forming constituents. One is to solely rely on antiscalant performance to keep the limiting constituents from precipitating out in the final stage of the RO membrane surface. RO systems using this strategy could achieve a recovery close to 90 percent. However, it stresses the membrane operation to its limit and represents a very high membrane scaling potential.
The second strategy is to use an ICCS process, such as lime softening, to drop out calcium carbonate, barium carbonate, sulfate, or magnesium hydroxide, and co-precipitate out silica. This process involves chemical and sludge handling, but greatly reduces the membrane scaling potential. Many research projects have successfully proven the effectiveness of the ICCS process in removing membrane scale-forming ions at bench scale. This strategy will allow the subsequent secondary RO membrane to achieve 60 to 75 percent recovery from the primary concentrate, exceeding an overall system recovery of 94 to 96 percent.
How It Works
Chemical techniques are known to be effective for removing both soluble and colloidal silica. These processes are often based on silica adsorption onto certain oxides and hydroxides, especially those formed with iron, alumina, and magnesium, i.e., Fe(OH)3, Al(OH)3, and Mg(OH)2. One proven process to remove silica through adsorption on magnesium hydroxide is to raise the pH up around 10-11. Another important silica removal mechanism through chemical precipitation is to use aluminum hydroxides in the pH range of 8.3 to 9.1. Activated alumina can remove silica effectively at pH 8 to 9.
Only a few pilot scale ICCS studies were completed and published. Major lessons learned from these studies can be summarized as follows:
- Particulate matter carried over from the clarification zone of the ICCS reactor. The insufficient settling results from an undersized clarification zone based on misused design parameters for conventional softening. This is typically seen in many commercially available softening skids.
- Most of the reported pilot reactors were designed in a conventional coagulation, flocculation, and sedimentation configuration without enough solids recirculation. Such recirculation is critical to process optimization because it provides precipitation nuclei, improves the particle size and settling characters, and facilitates chemical reactions. To optimize the removal of scale-forming constituents and minimize particulate matter carry-over, a solids contact clarifier should be used in lieu of the conventional coagulation-flocculation-sedimentation design.
- Lime fouling on pH probes impeded successful automatic pH control. Maintaining reliable automatic pH control and steady operation became quite challenging. A more robust on-line pH control strategy to attain consistent softened concentrate pH was recommended.
- Due to the difficulty in feeding, pumping, and handling
lime slurry at a pilot scale, many researchers ended up giving up on lime and used caustic soda (Sodium Hydroxide, NaOH) for pH adjustment. The differences between the two chemicals can be found in a later section of this article.
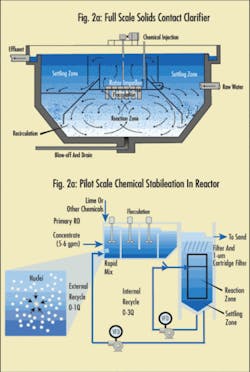
Many valuable design considerations were incorporated into the pilot-scale ICCS reactor design for a recent pilot study funded by the City of Phoenix. Figure 2 depicts the configuration of the lime softening reactor, designed and fabricated by the pilot study team.
The reactor mimics the function of a full-scale solids contact clarifier, which consists of a rapid mix zone, a flocculation zone, a bell-shaped reaction zone, and a settling zone. The total retention time of the reactor is about 40 minutes. The surface loading rate for the settling zone is 0.4 gpm/sf, about half of what a typical textbook would recommend for softening (i.e., 0.75 gpm/sf).
The reactor also includes an internal recirculation (0 ∼ 3Q) to recycle settled sludge back to the reaction zone to increase the chances of particle collisions and enhance the efficiency for chemical reaction. The external recirculation (0∼1Q) delivers settled sludge back to the rapid mix zone, where lime or other chemical is added. This recycle provides an effective seed for newly formed flocs to attach to.
City of Phoenix Case Study
To maximize water recovery and continue the development of reliable water supplies for its customers, the City of Phoenix recently implemented pilot testing on brackish water desalination and ICCS concentrate management. The ongoing pilot study demonstrated the feasibility of achieving 94 percent overall recovery on brackish surface water and groundwater using microfiltration surface water pretreatment skids, a primary RO system, an ICCS reactor, a sand filter, and a secondary RO system.
The two-stage primary RO system operated at 85 percent recovery, generating a 5.5 to 6.5 gpm concentrate stream with a TDS concentration of 5,000 to 10,000 mg/L. The concentrate was stabilized using a conventional “solids contact clarifier type” lime (or caustic soda) softening pilot reactor followed by a sand filter; this treatment system removed potential scale-forming constituents and particulate matter formed during the softening process. On average, the ICCS reactor and the sand filter removed 85 percent silica, 60 percent calcium, and 85 percent barium and produced a feed to the secondary RO system with turbidity less than 0.5 NTU and a Silt Density Index (SDI) less than 5. The single-stage secondary RO recovered 60 to 70 percent water from the stabilized concentrate with manageable scale formation.
Comparing Lime and Caustic Soda
Based on the City of Phoenix Western Canal Pilot Study data, differences between lime and caustic soda can be summarized in the following aspects:
- At the same pH, caustic soda removes slightly less but comparable levels of silica and barium, while lime often removes more calcium. Depending on specific water quality and levels of the limiting scale-forming ions, this could imply that potentially higher secondary RO recovery can be achieved on lime stabilized concentrate versus on the caustic soda stabilized concentrate.
- From an O&M and safety standpoint, caustic soda has the advantages of stability in storage, lower sludge formation, and easy handling. However, it is generally more dangerous to the operator by causing more severe burns to the skin.
- From a chemical cost standpoint, caustic soda is generally more costly, even though a lower chemical dose may be required to reach the same pH target. What is often neglected is the acid required for post-ICCS pH adjustment and secondary RO calcium carbonate control. Because lime removes hardness as well as alkalinity in the form of calcium carbonate, while caustic soda does not remove alkalinity, it often requires much more acid to lower the pH for the caustic soda stabilized concentrate.
The above comparison is highly dependent on the actual concentrate water quality. Conclusions may vary.
Low Energy Concentrate Volume Reduction
Proven as it is, the conventional softening-based ICCS reactor requires a significant amount of lime or caustic soda to raise the concentrate pH to above 10.5. The process generates a large volume of residuals, which in turn has to be handled properly. An ideal application for this process may be at a brackish surface water treatment facility, where a residual handling facility for the main stream surface water treatment is implemented and the additional volume of the residuals from the side stream concentrate management becomes fractional. Moreover, the blending of lime residuals may even enhance the settleability and dewaterability of the surface water alum or ferric sludge.
Literature review and preliminary bench scale data obtained at Arizona State University demonstrates that the ICCS can be modified into a high-rate pelletized softening reactor to reduce chemical usage, reactor footprint, and the volume of residuals. In addition, the overall water recovery may be improved. The modified ICCS reactor could be ideal for well head or centralized groundwater desalting facilities.
About the Author:
Charlie He, senior project engineer with Carollo Engineers in its Phoenix Office, has more than eight years of experience in water and wastewater treatment. Mr. He has a MS degree in Civil / Environmental Engineering from Arizona State University and a BS degree in Environmental Engineering from Tsinghua University, Beijing, China. He is experienced in Disinfection By-product evaluation and modeling and has gained extensive experience in membrane desalination and concentrate management in the recent years.