Use of Ion Exchange for NOM Removal Prior to MF/UF
By Michael Bourke and Miguel Arias
Natural organic matter (NOM) in water sources can cause numerous problems with the operation of membrane systems such as reduced flux rates and increased cleaning frequencies. The operational problems stem from NOM adsorption on the membrane surface combined with pore blockage, which ultimately fouls the membrane. This increases the energy usage required to maintain the same membrane productivity. In addition, a large fraction of dissolved NOM passes through microfiltration (MF) and ultrafiltration (UF) membranes, resulting in discoloration of the treated water, the formation of disinfection byproducts (DBPs) and biological growth in the distribution system.
To overcome problems caused by NOM at membrane filtration plants, utilities have implemented pretreatment using coagulation, either with or without sedimentation, and/or post treatment using granular activated carbon (GAC) adsorbers. In some cases, high pressure membranes such as nanofiltration or reverse osmosis have also been used to treat a portion of the MF or UF system product water to reduce treated water NOM levels. Ion exchange is an alternative technology that can allow significant improvements in downstream membrane performance and final treated water quality due to a reduced organic loading while also providing a less energy intensive option for NOM removal.
NOM Impact on MF/UF Performance
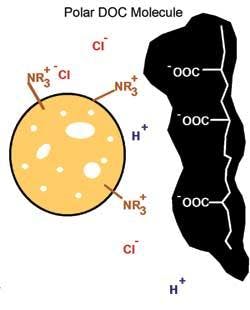
Natural organic matter can be characterized a number of different ways including separation by size, molecular weight (MW) and polarity. Size fractions can be defined as particulate (>0.45 µm), dissolved (<0.45 µm) and colloidal (~1 nm to 1 µm) where the colloidal fraction spans the dissolved and particulate definitions. The dissolved organic carbon (DOC) fraction can be further characterized in terms of polarity (negative charge).
The DOC fractions in order of increasing polarity are defined as hydrophobic (typically the largest DOC fraction for natural waters), transphilic and hydrophilic. Generally, hydrophobic DOC has the highest MW followed by transphilic and hydrophilic DOC fractions. While most DOC exhibits polar characteristics, there is a higher density of polar groups on smaller hydrophilic compounds compared to larger hydrophobic compounds.
Numerous studies, from laboratory to full-scale, have been conducted to investigate the impacts on membrane performance of different NOM fractions. Howe and Clark (2006) concluded that it is the dissolved and colloidal fraction of NOM that is responsible for most of the membrane fouling potential rather than particulate organic matter. While only a fraction of the DOC is responsible for most membrane fouling, it was determined that the overall ability to remove DOC was an effective predictor of the ability of coagulation to improve membrane performance. Other studies have identified that the hydrophilic fraction of DOC provides a larger contribution to membrane fouling than hydrophobic humic substances.
Coagulation is the method most commonly used to reduce the level of DOC prior to low-pressure membranes. The fraction of DOC preferentially removed by coagulation is the higher MW, more hydrophobic substances rather than lower MW hydrophilic substances.
Ion Exchange Pretreatment
As a large percentage of the DOC in potable water sources is polar (hydrophobic, transphilic and hydrophilic), any fraction of charged DOC can potentially be removed by anion exchange, although increased charge density increases affinity for the resin surface.
Anion exchange resins remove DOC by exchanging a chloride ion on the resin surface for polar dissolved and colloidal organic material. The resins can then be regenerated with sodium chloride solution. Numerous studies have shown that ion exchange preferentially removes high charge density, medium-to-low MW organic material, which can consist of hydrophobic, transphilic and hydrophilic organic fractions. Ion exchange can therefore be synergistic with coagulation in reducing DOC loadings onto membranes, where coagulation removes the lower charge density, higher MW hydrophobic fractions.
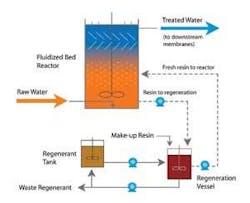
Traditionally, ion exchange has not been used for membrane pretreatment due to turbidity blocking the resin bed and biological activity fouling the resin. The development of mixed bed and fluidized bed ion exchange systems has now made it possible for this technology to provide DOC removal prior to membrane filtration.
The MIEX® Process uses a fluidized bed ion exchange reactor to remove DOC while allowing turbidity to pass through the reactor for downstream removal. A magnetized anion exchange resin is continually withdrawn from the reactor for regeneration and at the same time is replaced by fresh regenerated resin to maintain consistent removal of the target anion (DOC in this case) with no risk of chromatographic peaking (contaminant dumping). The continual regeneration of resin in brine also minimizes any biological growth within the ion exchange process.
Ion Exchange Pretreatment Case Studies
The MIEX process has been employed as pretreatment at a number of membrane filtration systems in Australia and the United States, primarily to reduce treated water DOC levels and therefore minimize the formation of disinfection by-products (DBPs). The process has been selected in preference to coagulation for membrane pretreatment due to greater removal of DOC as well as eliminating operator concerns about optimizing coagulant doses to accommodate fluctuations in feed water quality and temperature. Pilot data has also demonstrated a slower rate of trans-membrane pressure (TMP) increase using MIEX pretreatment both with and without coagulation.
Case 1: Improving Membrane Performance:
Figure 3 shows results from a pilot study that compared pretreatment to a Pall MF membrane with the MIEX process followed by in-line coagulation versus in-line coagulation only. The graph illustrates the rate of TMP buildup at a constant flux for ion exchange/coagulation pretreatment and coagulation only pretreatment. With ion exchange/coagulation pretreatment, it took approximately 11 days for the TMP to increase from 6.0 to 9.0 psi. After 11 days the ion exchange pretreatment was turned off while the coagulant feed was maintained at the same dosage (15 mg/L). The TMP increased from 7.6 to 49.3 psi over the next three days, indicating that ion exchange pretreatment was stabilizing the coagulation process performance.
Optimizing coagulant dosing without ion exchange pretreatment required an increased coagulant dose (20 mg/L) and a similar TMP increase (5.2 to 10.7 psi) that took 11 days to achieve with ion exchange pretreatment occurred in less than two days. The raw water total organic carbon (TOC) level of 4.6 mg/L was reduced to 2.9 mg/L with coagulation only and to 1.3 mg/L with ion exchange/coagulation treatment. These results indicate that TMP increases can be severe if coagulation pretreatment is not optimized for raw water quality. Coagulation does remove fouling portions of organic matter yet coupled with anion exchange, additional fouling fractions are removed providing a significant improvement in membrane performance.
Case 2: Reducing DBP Formation:
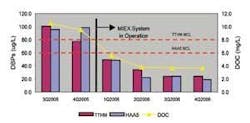
A water treatment plant at Big Elk Meadows, CO, treated surface water from a local lake using a Siemens MF system followed by GAC adsorbers. Raw water DOC levels ranged from 9 to 16 mg/L and the treatment plant only reduced the DOC by 1 to 2 mg/L. Disinfection of the resulting treated water resulted in TTHM and HAA5 levels well above the EPA Stage 1 Disinfection Byproduct Standards.
Options investigated to reduce treated water DOC levels and the subsequent DBP formation included more frequent replacement of GAC, the installation of a packaged coagulation/clarification system and installation of a MIEX ion exchange system prior to membrane filtration. More frequent GAC replacement was ruled out due to operational cost and labor required and a coagulation/clarification system was ruled out because of the high level of operator input and skill that was necessary to operate this system in addition to cold water constraints of the coagulation process.
The ion exchange system was chosen as a pretreatment step to remove DOC prior to the membranes based on the simplicity of operation and low operating costs compared to the alternative options. The removal of over 70% of the raw water DOC provided by anion exchange resulted in a similar percentage reduction in distribution system TTHM and HAA5 levels, allowing the water system to come back into compliance comfortably below EPA Standards.
Conclusion
Tightening EPA DBP standards and an increasing need to use poorer quality water sources for drinking water is increasing the need to remove as much DOC as possible prior to membrane filtration. Coagulation has typically been used to remove particulates and some DOC prior to membranes but the ability to remove DOC is limited. The introduction of new ion exchange system configurations has allowed ion exchange to be used alone or in conjunction with coagulation to provide greater removals of DOC and foulant fractions, which can significantly improve the performance of the membrane system while producing a higher treated water quality. —m
References
1. Howe, K; Clark, M; “Effect of coagulation pretreatment on membrane performance”, Journal AWWA, April 2006.
About the Authors:
Michael Bourke is a Vice President of Orica Watercare Inc. located in Denver, CO. He has worked for Orica Watercare in Australia and the U.S. since 1989 in the development and commercialization of new water treatment technologies. He can be reached at [email protected]. Miguel S. Arias is a Water Treatment Specialist for Orica. He received his PhD from the University of Colorado at Boulder in 2005 where his focus was water treatment process optimization. His industry experience has ranged from sustainable potable water projects in Tsunami affected regions of Indonesia to optimizing membrane performance and minimizing disinfection byproduct formation using ion exchange for North American Utilities.