Study Examines Impact of Desalination Pretreatment on Organic Matter
Pretreatment for seawater desalination typically focuses on removal of particles but many of the problems with membrane fouling are due to natural organic matter in water. American Water conducted a study to evaluate the fate of organic carbon in desalination pretreatment processes, which specifically concerned the removal of assimilable organic carbon (AOC) and total organic carbon during desalination pretreatment.
By Orren D. Schneider, Lauren Weinrich, Mark W. LeChevallier, and Eugenio Giraldo
Pretreatment for seawater desalination typically focuses on removal of particles but many of the problems with membrane fouling are due to natural organic matter in water. Fouling of reverse osmosis (RO) membranes is one of the major concerns in the desalination industry. High fouling rates lead to either increased pressure to maintain a permeate flux set-point or decreased specific flux, which in turn lead to higher capital and operations and maintenance costs.
To reduce fouling, many desalination facilities include coagulation as a pretreatment step designed to remove particulates and organic matter and limit biological growth in the membrane systems. However, little literature exists regarding how these pretreatment processes are optimized for reducing foulants.
At present, the main tool that is used for evaluation of RO membrane fouling is the silt density index (SDI) or its close relative, the modified fouling index (MFI). However, the SDI (or MFI) is based on the plugging of a 0.45-micron membrane over a defined time interval (often 15 minutes) and is not truly representative of RO membranes characteristics and properties; nor do the SDI or MFI tests account for biological growth on membranes or membrane systems, or take adsorption of organic matter onto membrane surfaces into account.
The main causes of membrane fouling on surfaces or inside pores are biological growth, deposition of existing or precipitated particles, and adsorption of organic matter. Although micro/ultrafiltration membranes are being used more commonly as pretreatment for reverse osmosis and nanofiltration membranes, submicron particles and dissolved organic matter can still pass through the pretreatment membranes and act as foulants on the RO membranes. Removal of the foulants can be improved by optimization of coagulation as a pretreatment.
For surface waters, tools to evaluate coagulation and biogrowth potential have been developed and have been used for design and treatment optimization in many places. However, for technical and economic reasons (traditional particle surface charge analyzers do not reliably operate in high ionic strength water and traditional AOC methods cost >$400 per sample), the desalination industry has not used these same diagnostic approaches in the design/operations of desalination facilities. Therefore, reliable operation of coagulation resulting in stable, high quality feed water for reverse osmosis membranes has been very difficult to achieve.
American Water conducted a study to evaluate the fate of organic carbon in desalination pretreatment processes, which specifically concerned the removal of assimilable organic carbon (AOC) and total organic carbon during desalination pretreatment.
In order to accomplish this objective, the research followed several distinct but interrelated tasks. The first was the development and refinement of a method to measure AOC in salt water to enable more timely evaluations of biofouling potential. This method was developed using a naturally occurring marine bacterium (Vibrio harveyi). The method was refined in the lab using both natural and model waters.
Following development of this method, a model for the fate of AOC and its impact on fouling was created using operational data from the Tampa Bay Seawater Desalination Facility, a desalination plant operated by American Water that has been challenged by increased fouling rates.
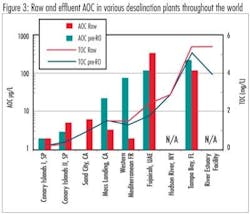
Because this was the first time that AOC has been measured in seawater using natural conditions and organisms, some context was needed to understand the results. In order to provide this context, samples were collected from various points in the treatment trains from seven desalination plants with a wide geographical range. Several of these plants had been identified as being subject to biofouling. These samples were analyzed for AOC and total organic carbon (TOC) to examine the fate of organic matter through these plants.
The last major task was a series of jar tests designed to identify coagulation conditions (coagulant type, dose, and pH) that could be used to improve organic removal in the pretreatment step of two desalination plants (The Tampa Bay facility and a pilot plant on California's Monterey Bay operated by California American Water). When these tests showed that the plants were achieving the maximum organic removal for feasible operations and their design, additional jar tests were conducted to examine what design and/or operating conditions would be required to achieve substantially higher organic removal.
An analytical method was developed that is useful for measuring AOC in saline waters. The method uses V. harveyi, a naturally occurring marine organism that can use a diverse range of organic substrates. Additionally, the development of the method has shown that the emitted light in the luminescence-based test was directly proportional to the biomass of the AOC organism in a sample; thus this new method can provide a lower cost alternative to monitoring biomass through traditional plate count methods. Because the test equipment can be automated to read the luminescence of multiple samples, the method provides both the maximum growth potential (AOC) of the water sample as well as the substrate uptake rate (μmax) of the organic matter in the water. The test showed applicability to a wide range of raw and treated saline water sources. The utility of the marine AOC test was apparent when conducting both inter- and intra-facility testing where this assay allowed comparisons of AOC across treatment trains to examine the fate of AOC in different treatment processes.
A mathematical model was developed for the Tampa Bay Seawater Desalination Facility to examine the factors that lead to increased pressure drops along the pressure vessels. The model included elements to account for flow rates, AOC concentration, and substrate utilization rates. Using data from a single train of the plant (representative of the entire plant), the model was calibrated to match existing operational data. The development of this model showed that increased pressures in the pressure vessels can be almost entirely explained by bacterial growth (biofouling) and thus points to the importance of AOC control through pretreatment. However, no direct correlations have been made between AOC concentrations and fouling rates.
Samples were collected from nine pilot or full-scale desalination facilities around the world. Based on these field samples, it was observed that the range of AOC and TOC in saline source waters varies widely. AOC in the raw waters ranged from <5 μg/L to nearly 500 μg/L; the TOC in raw water ranged from <1 mg/L to >10 mg/L. Results from this study indicated that plants that use beach wells had lower raw water organic levels, i.e., AOC and TOC, than plants that use open water intakes. Consistent reductions in both AOC and AOC substrate utilization rates after the beach wells indicated higher quality water and a reduced potential for biological fouling. Pretreatment at these desalination plants was found to be generally effective at removing AOC. Removal of TOC by pretreatment was generally poor (<10%), the one plant that achieved significant TOC removal uses brackish river water as the source and thus, the raw water has significantly lower salinity than the other plants examined.Jar testing of plant inlet water from both Tampa Bay and Monterey Bay has shown that, under coagulation conditions used for control of SDI, little TOC removal was achieved. Results from field samples collected at other desalination plants showed that TOC removal through pretreatment was generally poor. When applying "extreme" coagulation conditions in jar tests (ferric chloride doses up to 100 mg/L, oxidation with ozone up to 2 mg/mg TOC, or coagulation at pH 5), organic removal was increased; however, the benefits of increased organic removal on membrane fouling would have to be weighed against increased chemical costs and increased sludge production.
It was found during the jar testing that the untreated seawater tested contained a substantial number of positively charged particles. This differs from fresh waters that contain virtually no positively charged particles. This would seem to indicate that, although electrodynamically unstable, the particles were nonetheless stable in suspension. This was likely due to steric stabilization, probably caused by organic matter adsorbing to particle surfaces and preventing the particles from approaching close enough for attractive van der Waals forces to dominate.
Investigations using model waters showed that as the salt concentration of water increased, the zeta potential of particles became less negative. When sodium chloride (NaCl) was used, no positive colloids were formed, even at NaCl concentrations of 25,000 mg/L (approximately equal to the total dissolved solids – TDS of estuary water). When a commercially available seawater mix was used, positive colloids were found at high salt concentrations (25,000 mg/L). This would seem to indicate that polyvalent cations (mostly magnesium and calcium) played an important role in the formation of these positive charges, probably due to complexation of the cations by organic functional groups.
Jar testing with model waters showed that when sodium chloride was used as the background ionic matrix, higher salt concentrations substantially increased coagulation performance by reducing the coagulant dose required for removal of turbidity and organic carbon. When the artificial seawater was used, low (500 mg/L) and moderate (5,000 mg/L) salt levels improved turbidity and organic carbon removal. However, at salt concentrations that approximated seawater (25,000 mg/L), organic carbon removal was retarded compared to the lower salt and no salt conditions. When comparing TOC removal in the model waters, at all coagulant doses tested, the percent removal of TOC was lower when the artificial seawater was used as compared to when sodium chloride was used as the ionic matrix.
In conclusion, the results of these studies, suggest that when terrestrial organic matter enters highly saline water, it undergoes complexation by magnesium and/or calcium to form soluble complexes that control its subsequent surface chemistry. When absorbed onto silts, these complexes become difficult to remove by charge neutralization and may require separation by enmeshment requiring high coagulant doses or coagulation at extremes of pH that impact the surface charge and allow for removal.
About the Authors: Orren D. Schneider, Ph.D., P.E., is Senior Environmental Engineer at American Water where he is involved with research leading to optimization of water treatment and distribution system operations. Lauren Weinrich is a Senior Research Analyst at American Water and her current research areas include seawater RO membrane pretreatment and biological stability in drinking water distribution systems. Mark W. LeChevallier, Ph.D., is currently the Director of Innovation & Environmental Stewardship at American Water. Eugenio Giraldo, Ph.D. is the Technical Manager for wastewater commercial projects at American Water where he provides technical support to wastewater operations across North America.
More WaterWorld Current Issue Articles
More WaterWorld Archives Issue Articles