Analytical instrumentation monitors and maintains plant efficiency
The proper use of analytical instrumentation in thermal and membrane desalination processes ensures water production costs remain low and plant efficiency is maximised.
Fresh water is rapidly becoming the world's most precious resource. Too many people live in places with too little fresh water so it makes sense that as the cost of producing drinking water has increased and the cost of treating seawater and brackish water has decreased, the interest in desalination as a viable solution to supply the world with a safe and reliable source of drinking water is on the rise.
Desalination is used in more than 100 countries and has had a big impact in parts of the world where lack of fresh water would otherwise limit population growth, and industrial and agricultural development. Desalination has also benefited from a synergy with power plants since these plants are often located by the ocean and already have extensive piping that draws water into the plant for cooling. Using these same resources for desalination makes both applications more cost effective. More than half of the desalination performed today is in the Middle East and North Africa, with 24% in Saudi Arabia alone, and the United States ranks second in the world at 16% and rising. The desalination market is predicted to double in the next 15 years - an increase to a value of over $70 billion.
Several technologies that can be used for desalination are divided into thermal processes including all forms of distillation, and membrane processes including electrodialysis and reverse osmosis (RO). Today, reverse osmosis and multi-stage flash distillation are the dominating trends. Because distillation tends to be an energy-intensive process, it is mostly used in areas where energy is readily available, such as the Middle East. RO, which requires less energy, is commonly used in western countries. Regardless of which technology is used, however, the maximisation of plant efficiency, equipment life and uptime is, to a great degree, a function of good water chemistry. And monitoring good water chemistry relies on the proper use of analytical instrumentation.
Water analysis in thermal processes
Nearly half of the world's desalinated water is produced by distillation. This process closely mimics the natural cycle of water in that salt water is heated to produce water vapour that then condenses to form fresh water. Performing distillation economically requires that the pressure of the water be adjusted to control the boiling point (the temperature required to boil water decreases as pressure lowers). Reducing the atmospheric pressure on the water being boiled allows multiple boiling of the same water in multiple vessels, each time at a lower pressure and temperature. This reduces the amount of energy required for distillation and increases the yield of fresh water.
Controlling the boiling point of the seawater not only allows multi-stage boiling but also affects another key issue in desalination - the formation of scale. Carbonates and sulphates are readily dissolved in seawater. They begin to leave solution when the water approaches 95o C and forms a hard scale. This scale coats any tube or surface creating enormous thermal and mechanical problems in the desalination system. Since this scale is difficult to remove, preventing the formation is the key to success, which can be done by controlling the concentration level of the seawater and restricting the higher process temperatures. In addition, acid chemicals can be added to the seawater that reduce scale precipitation and allow the temperature to reach 110o C. Operating a plant at these higher temperatures enhances efficiency, but too much acid has the potential to cause corrosion.
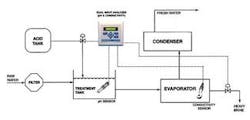
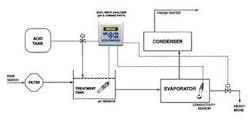
Figure 1 shows a typical seawater distillation layout. The raw seawater is filtered to remove solids and then combined with acid in a treatment tank. The treated seawater is heated in an evaporator where much of the scaling can occur. The resulting vapour is collected and recondensed in the condenser as fresh water, and the salt from the seawater is removed as brine.
Two separate scaling problems must be dealt with in the evaporator. The first problem includes calcium carbonate (limestone) and magnesium hydroxide scale, which are formed under higher pH conditions when bicarbonate ions can form carbonate ions and when hydroxyl (OH) ions are present. Carbonate and hydroxyl ions react with the calcium and magnesium normally present in the seawater. Lowering the pH of the feedwater to less than 5.7 can minimise calcium carbonate and magnesium hydroxide scaling. This pH reduction is commonly achieved by adding citric acid, ferric chloride or sulphuric acid. Since too much acid will result in corrosion, the precise pH balance must be maintained with the use of a pH sensor and analyzer. Typically a pH sensor will be placed in the treatment tank as shown in Figure 1.
A second scale problem appears when the feed solution becomes saturated with calcium sulphate. Calcium sulphate can't be controlled by pH adjustment. The concentration of calcium sulphate must be maintained below the saturation point (roughly 2 grams per litre) by removing a portion of the heavy brine in the evaporator. The concentration can be monitored through conductivity measurement. Although conductivity doesn't provide a specific indication of the concentration of calcium sulphate, it does correlate to the level of all dissolved solids. A conductivity sensor with a toroidal design reduces sensor-fouling problems in this environment.
Water analysis in RO
Reverse osmosis is a relatively new desalination technique having only been commercialised in the 1970s. It employs a membrane separation process in which water from a pressurised saline solution is separated from the dissolved solids by flowing through a membrane. Unlike distillation, heat is not required. The saline feed water is pumped into a closed vessel that is pressurised against the membrane. A portion of the water passes through the membrane while the remaining water increases in salt content. A portion of the saline feed water is discharged without passing through the membrane in order to reduce this salt concentration.
The key factor in the success of RO is the "care and feeding" of the semi-permeable membrane, especially since this membrane can represent a considerable capital investment, and frequent replacement is costly and time intensive. If the membrane becomes clogged or is attacked by chemicals or organisms it can significantly reduce the success of the desalination operation. For this reason, RO generally requires pretreatment to remove suspended solids and assure that salt precipitation or microbial growth doesn't occur on the membranes. Pretreatment usually consists of fine filtration and the addition of acid or other chemicals. As in distillation systems, however, the chemicals can cause as many problems as they prevent if not properly monitored.
Efficiency of the RO membrane can be monitored through the use of conductivity measurement. The efficiency is the amount of dissolved solids content of the product water divided by the volume of feed water. In a new system this efficiency number could be between 2% to 5% passage (or 98% to 95% rejection). (Percent rejection is the difference between the conductivity of the feed water and permeate divided by the conductivity of the feed water.) Eventually the efficiency of the membrane will decrease to the point that it must be replaced. As shown in Figure 2, one conductivity cell, called the feed water cell, is placed on the RO input. A second conductivity cell, called the product cell, is placed on the outlet. A conductivity ratio analyser measures the conductivity of each cell and calculates the percent passage or percent rejection. These analysers are equipped with alarms to monitor the ratio and the product water conductivity, allowing the operators to know when membrane attention is required. General-purpose conductivity instruments also provide temperature readings. (Conductivity is a function of temperature, so accurate temperature-corrected conductivity measurement is important.)
The final discharge from an RO desalination plant is a concentrated brine solution. To measure this concentration, plants can use conductivity to measure the salt concentration of plant discharge in order to meet environmental standards.
Cellulose acetate RO membranes are not recommended for salt-water desalination, but if the feed source is brackish water, a cellulose acetate RO membrane can be used. These membranes tend to be degraded by alkaline (high pH) water, resulting in a loss of efficiency. In feed water with calcium hardness, precipitation (scaling) can occur on the sample side of the membrane in alkaline pH ranges due to the concentration of dissolved solids. To protect the membrane and avoid scaling, the pH of the feed water can be adjusted to the acid side with a target of pH 5.5. The control action is not difficult since the feed water doesn't typically tend to have major pH fluctuations or load changes (changes in the titration curve). Again, a general-purpose pH sensor will assure that pH remains acidic. Placement of the sensor in the feed water stream is shown in Figure 2. To accommodate changes in flow rate, a flow measurement can be used to trim the pH control.
In total, providing reliable, efficient liquid analytical measurements to assure water quality in desalination systems is simple and cost-effective. The important step for the plant operator is recognising the critical importance of choosing the right instrumentation and sensors to monitor the desalination process, and assure that costs are kept low and plant efficiency is maintained. Continued development and improvements in the various desalination treatment methods and the making of liquid analytical measurements will have a significant impact on our ability to provide a future source of safe and reliable drinking water for our growing population and our industrial needs for fresh water.
Author's Note
John Volbeda is the industry manager of water and wastewater for Emerson Process Management, Rosemount Analytical. Liquid Division.