AFM, Glass Media and Sand Filtration Systems
By Dr. Howard Dryden
Media bed mechanical sand filtration systems comprise gravity flow, pressure and moving bed continuous backwash filtration systems. In all cases the most common mechanical filtration media is quartz silica sand. The quality of quartz sand is a variable depending upon the country and the location of the deposit. There is a requirement for a consistent quality of filter media for all industries using media bed filtration in order to standardize and optimize the filtration process. Applications include drinking water treatment, pretreatment prior to membranes, industrial process water and tertiary treatment of wastewater. Filter media quality is critical for filters that have a pressure gradient across the bed, such as horizontal filters, or filters that have not been installed on a perfectly level base.
History
Sand has been used for over 200 years in Europe as a means of filtering drinking water. A company in Scotland in 1804 was the first documented report of a company using sand in a slow bed sand filter.1 Slow bed sand filters typically operate at a water flow velocity of 0.1 m/hr and use a coarse grade of sand and gravel. The filters depend on maturation of the sand as a biological filter before they provide adequate mechanical water filtration.
Flow Rates
Slow bed sand filters provide excellent water quality and are still used for the treatment of drinking water. Approximately 15 percent of all water supplies in the UK currently use slow bed filters, but they are being phased out in favor of Rapid Gravity Filters (RGF) and pressure sand filters in order to save space. RGF filters for drinking water operate at a water flow velocity of 6 m/hr whereas pressure filters typically operate at 12 m/hr. The water flow velocities of RGF and pressure filters are therefore 60 to 120 times faster than slow bed filters. The higher water velocities change the biodynamics of the filtration process, which impacts filter performance leading to bio-instability and transient wormhole channeling of unfiltered water through the filter bed.
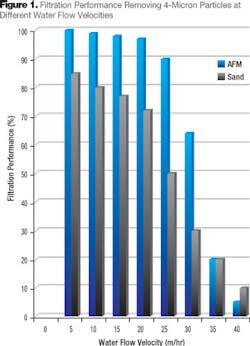
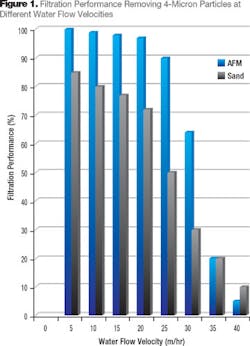
The performance of any media bed will be inversely proportional to the flow velocity, which is a function of the filter diameter, its surface area and bed depth (Darcy’s Law).2 One of the key issues in the drinking water industry is ability to remove Cryptosporidium, which is almost completely resistant to chlorine and only measures 4 microns in size. Figure 1 compares the performance of activated filter media (AFM) and Leighton Buzzard sand from England. The slower the filter flow velocity the higher the performance. The relationship is exponential but the coefficient depends on the media characteristics and particle size used for performance evaluation.
Water treatment systems tend to operate at the highest possible water flow rates in order to save space and reduce capital cost. AFM has been shown to provide performance advantages over sand, which permits higher water flow rates and reduced capita cost of installations. Typically 25 percent higher water flow rates can be used with AFM over sand while still maintaining a better performance.
Filtration performance also depends upon filter configuration. Horizontal filters save space and maximize surface area, and bed depth is shallower, which reduces adsorption capacity for small particles. Also, a differential pressure gradient across the bed reduces performance when compared to vertical filters that have a consistent pressure gradient and a deep bed. The differential pressure promotes biofouling of sand, biodynamic instability and transient wormhole channeling; these problems are largely resolved by using AFM, which does not suffer from biofouling.
Pressure Differential
During the run-phase large solids will accumulate on the top of the filter bed and small solids will penetrate the bed. Small particles attracted by electrical (Van der Waals) forces may become trapped on the surface of the media. Sand and most media carry a negative charge or zeta potential. In water treatment, coagulants and flocculants, such as Lanthanum chloride, aluminum chloride, ferric chloride, polyaluminum chloride (PAC) or polyelectrolytes, may be applied to drop the zeta potential, increase coagulation and flocculation, as well as increase electrical attraction. In some applications, including pretreatment prior to membranes, the use of chemicals would not be advisable. Reduction of zeta potential and coagulation can nevertheless be achieved by the rapid movement of water using cavitating static mixers such as a zeta potential mixer (ZPM) or by slightly increasing redox potential by application of ozone.
In addition to mechanical and electrical attraction, there will also be some degree of molecular sieve filtration. This will be the case with activated carbon, and to a lesser extent with new sand. The ability of sand to adsorb is a function of the silicon-to-aluminum ratio and how the molecules are configured. An example of natural ion exchange molecular sieve sand is the zeolitic sand clinoptilolite.3,4
Zeolites are used in water treatment as a mechanical filtration media and also as an ion exchange mineral for the selective removal of ammonium and radioactive nucleotides from fresh water. Zeolites cannot be used for marine systems or water with a high TDS because the competing cations will prevent ion exchange. In freshwater systems zeolites provide a good substrate for the growth of autotrophic nitrifying bacteria, a characteristic that is likely due to the adsorption of ammonium into the mineral and its availability to be metabolized by autotrophic species such as Nitrosomonas spp.
Biofouling and Wormhole Channeling
The performance of any mechanical filtration media depends upon the passage of water through the filter bed and the ability to remove the collected solids during the back-wash phase. The zeolite clinoptilolite initially provides very good filtration, but the media rapidly biofouls, especially at high water temperatures (above 20°C) in nutrient-rich water. The rapid growth rate of bacterium and the production of bacterial alginate exopolysaccharides cause coagulation of the filter bed5 which results in transient wormhole channeling. The alginates are actually advantageous in slow bed filters6 and can improve filtration performance. In rapid gravity and pressure sand filters the alginates lead to blockage and bio-instability of sand beds. Backwashing will not remove biofilm or prevent biofouling. Indeed, continuously fluidized sand beds make excellent biofilters for bacterial nitrification.7
Backwashing: What Goes In Must Come Out
Proper backwashing is very important. Under German DIN standards the bed should be fluidized and expanded by 20 percent for a period of 5 minutes. The velocity of the water required to achieve the required bed expansion is a function of the bulk bed density of the media, particle size, and shape, as well as the temperature and density of the water. Sand with a particle size distribution (PSD) between 0.5 and 1.0 mm requires a flow velocity in the region of 55 m/hr at 28°C for fresh water.
Backwashing is critical; any solids or organic matter remaining in the filter bed after a backwash will simply act as a food source for the growth of more bacterium and production of exopolysaccharides. However, we know that even sand beds fluidized 100 percent of the time make very good biofilters,8 so backwashing of sand is never 100 percent effective. Organic matter and particles will become embedded in the alginate and will remain after a backwash and will continue to feed heterotrophic bacteria. Gradually the sand biofilm layer will mineralize with calcium, magnesium, ammonium and phosphate to form calcites or struvite. The biofilm becomes more stable, alginate production increases and filtration performance gradually decreases until a point when a media change is required.
Glass Media and Sand
Glass is an aluminosilicate manufactured from silica sand or from the re-melt of glass bottles. It has a similar chemical composition to sand but may contain metal oxides such as aluminum or ferric (from amber glass) or manganese and chromium (from green glass).
In 1984, I used glass as a filter media alternative to the zeolite clinoptilolite as a means of filtering water in a recirculating aquaculture system (RAS) for eels and Atlantic salmon. The glass was initially used as a feedstock for the manufacture of synthetic zeolites. The glass was subsequently used as a substrate and the surface of the glass was changed by a solgel process to give it a hydrophilic high surface area to avoid biofouling while still acting as a molecular sieve similar to clinoptilolite for the adsorption of organics.
The manufacture of filter media provides an opportunity to make a filter media with a specific, tailored performance. The performance can then be quantified and compared against other filter media. Such an investigation has never been conducted for sand. Given that sand is used to treat more than 99 percent of our drinking water supply, it is rather surprising that there has been no detailed comparison of sand media performance from different deposits or different countries.
IFTS Testing
The Institut de la Filtration et des Techniques Séparatives (IFTS) is recognized as being the leading institute in Europe for the testing of water filter technology. As part of the development of a new International ISO 14034 standard for Environmental Technology Verification (ETV) of product performance in the water industry, glass media from different manufacturers in Europe were evaluated by IFTS. The three basic tests conducted included: run phase efficiency; injected mass test; and backwash performance.
Run Phase Particle Size Removal
Seven different types of glass media and one sand media were tested. The sand was from the Leighton Buzzard deposit in England. The silica sand was recognized by IFTS to be one of the best in Europe. The glass media was provided by different manufacturers of glass granules and glass beads in Europe.
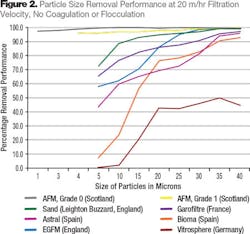
The run phase performance test involved the injection of particles of a known particle size directly into the water under controlled conditions. Particle size analyzers were fitted to the test rig in order to check the concentrations and confirm the performance. Two grades of AFM were tested: Grade 0 is fine-grade media with a PSD from 0.25 to 0.50; Grade 1 AFM is typical of most filter-grade media with a PSD of 0.4 to 1.0. The PSD of all the media tested approximated to a standard 16 x 30 mesh size.
The test was performed in a 150 mm diameter column with a 900 mm bed depth at a flow velocity of 20 m/hr and temperature of 23°C. At 5 micron particle size, AFM Grade 1 was removing more than 97 percent of all particles and sand was removing 72 percent. Vitrosphere filter media, manufactured from glass spheres, showed zero particle removal at 5 microns (see Fig. 2).
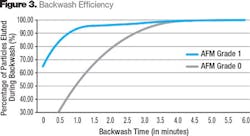
A mass balance was conducted on the data, as it is very important to achieve as close as possible to a 100 percent backwash efficiency. All of the non-activated glass filter media and sand showed a reduced backwash performance with 10 to 50 percent of solids remaining in the filter bed. AFM achieved 100 percent backwash performance after 5 minutes (see Fig. 3).
Summary and Conclusions
The performance of a mechanical filtration system will depend on the quality of the media, the design of the filter and the operating criteria. For best performance and water clarity with AFM, vertical pressure or RGF filters should be used. The run phase should be less than 20 m/hr and differential pressure should never exceed 0.4 bar. It is also best to backwash the media at least once a week at a water flow that fluidizes the bed by more than 20 percent for a period of 5 minutes, or until the backwash water runs clear.
There is a wide choice of filter media available. The sand tested was the best sand available, and the best performing filter media as shown by IFTS data was AFM activated filter media. The results reflect the performance of new sand; as sand ages, it will gradually become a biofilter and mechanical filtration performance will deteriorate over the following months.
The clarity of water is a function of the zeta potential of all particles in suspension. As redox potential increases, zeta potential decreases. When zeta potential is zero you have the lowest turbidity. Prior to filtration the water should have a redox potential over 300 mv; this may be achieved by aeration of the water. For manganese removal the potential should be 500 mv. In addition to aeration, chemicals such as ozone or chlorine dioxide may be required to remove manganese.
We recommend the use of a ZPM prior to filtration. The cavitating ZPM static mixer will drop the zeta potential and can raise the redox potential. The injection of cationic coagulants and flocculants such as All-Polyfloc (APF) will drop the zeta potential, clarify the water, increase redox potential and allow AFM to remove micron and sub-micron particles and even chemicals from solution. AFM Grade 0 will remove >99.7 percent of all particles down to 3 microns; AFM Grade 1 will remove more than 97 percent down to 5 microns. However, when AFM Grade 1 is combined with a ZPM, coagulation and flocculation, it will nominally remove most particles down to 0.1 micron, as well as much smaller particles and even chemicals from solution.
About the Author: Howard Dryden has a PhD in marine biology, specializing in water treatment using molecular sieve ion exchange filtration with the zeolitic sand clinoptilolite. Dryden Aqua was formed on the basis of applied research and the development of a novel water treatment process, which includes AFM, an activated filter media to replace sand.
References
1. “Filtration of Water Supplies,” World Health Organization. http://www.who.int/water_sanitation_health/publications/ssf2.pdf
2. Darcy, H. Les Fontaines Publiques de la Ville de Dijon, Dalmont, Paris, 1856.
3. Dryden, H. T. and L. R. Weatherley. “Aquaculture treatment by ion exchange: II. Selectivity studies with clinoptilolite at 0.01N.” Agricultural Engineering, 6: 51-68, 1987.
4. Dryden, H. T. and L. R. Weatherley. “Aquaculture water treatment by ion-exchange: I. Capacity of Hector clinoptilolite at 0.01-0.05N.” Agricultural Engineering, 6: 39-50, 1987.
5. Dryden H.T. “The removal of ammonium by selective ion exchange filtration using the natural zeolite Clinoptilote,” Heriot Watt University, Dept. of Chemical Engineering. 1984.
6. Law, S., et al. “Visualisation of the establishment of a heterotrophic biofilm within the schmutzdecke of a slow sand filter using scanning electron microscopy,” Biofilm, Volume 6, Paper 1 (BF01001), 2001.
7. Losordo, T.M., et al. “Recirculating Aquaculture Tank Production Systems: A Review of Component Options,” Southern Regional Aquaculture Center, Publication No. 453, April 1999.
8. ibid.