Low-level analysis of rare earth elements
Infinitely useful, yet economically difficult to extract, rare earth elements (REEs) are the group of gleaming, silvery transition metals located in the d- and f-blocks of the periodic table.
REEs include scandium and yttrium, plus a group of 15 elements, known as the lanthanides, often found clustered together in ore deposits. Despite their name, REEs are not rare in nature, but they are finite and are often difficult to extract in economically viable forms. However, their unique usefulness in modern technology, from medical diagnostics equipment to consumer electronics and renewable technology, makes them highly covetable and increasingly valuable.
There is, however, a darker side to REEs, exhibited mainly through their extraction and disposal. They are known to bioaccumulate1 and to persist in the environment2, raising concerns around their toxicity for both nature and humans in and around extraction and disposal sites. These concerns are certainly valid, as liver decline has been reported in humans in areas with REE pollution3. In response to the growing concern around REE contaminants, governments are starting to take action — but this is not without its complications. The clean-up project in one retired REE mining site in southern Jiangxi Province, China, has prompted international political wrangling around who should foot the $5.5 billion bill amid growing concerns about unregulated mining sites4.
To detect environmental pollution and ensure the safety of communities living in and around sites that mine or dispose of REEs, there is a clear need for reliable techniques to identify and quantify these elements.
On the Hunt for Rare Earth Elements
REEs need to be identified and quantified in order to detect environmental incidents and hazards, protect against bioaccumulation and safeguard drinking water supplies from contamination. However, detection difficulties often hamper this work.
Analytical laboratories have well-established methods for the identification of common analytes, such as chromium, arsenic, selenium, cadmium, mercury and lead, but REE analysis is not often a part of these standard processes. Instead, REE analysis is often confined to environmental research studies. The reasons for this are multi-faceted but mostly stem from the fact that REEs are often detectable only in trace, nanogram-per-liter levels. In addition to this already significant challenge, REEs are usually found in samples with widely variable compositions, often containing many elements of this group together with common analytes. This can result in complex matrices that introduce significant spectral interference. Polyatomic and isobaric isotopes can present overlapping results making it hard to elucidate REE elements from their counterparts and other transition metals. A doubly charged ion, such as neodymium 150Nd++, can easily be interpreted as a single positive arsenic ion 75As+ and the formation of gadolinium oxide 156Gd16O+ could be mistaken for the element ytterbium 172Yb+.
Kinetic energy discrimination is a common way to remove polyatomic interferences but is unable to overcome all interferences reliably. Other techniques, such as high mass resolution or reaction chemistry, can remove interferences but are often unavailable in analytical testing laboratories. Even after the considerable effort needed to run these techniques, they still might not deliver the precision needed to accurately resolve spectral interference and enable detection at such ultra-low levels.
The importance of REE detection, and the considerable challenges it brings, has not gone unnoticed – a new standard from the International Organization for Standardization (ISO) has recognized REE detection standards on a global scale. The organization’s latest standard, ISO 17294, in turn contributes to the United Nations Sustainable Development Goals. Specifically, ISO 17294 prescribes the standardized methods needed to measure REEs in surface and drinking water. These standards are accepted by the Europe Union and many other regulatory bodies.
Triple Quadrupoles Cut Through the Interference
A key focus of ISO 17294 is the recognition of inductively coupled plasma mass spectrometry (ICP-MS) as the standard for REE detection in surface and drinking water. In recognizing a method that is already widely used in the analysis of common analytes, ISO 17294 compliance doesn’t require the addition of expensive new equipment or complex new workflows. Water analysis laboratories can adopt this new standard quickly and easily to provide value-added REE analysis services and gain a competitive advantage.
The reason for the ISO 17294 adoption of the ICP-MS method seems clear. ICP-MS delivers a fast, sensitive and robust method for the detection of low concentrations of REEs with even the most complex matrices. This powerful elucidation capability lies in the heart of ICP-MS technology: the triple quadrupole and its ability to remove spectral interference.
The first quadrupole removes all ions, except for a specified mass-to-charge (m/z) ratio; next, the interference caused by polyatomic and isobaric ions is removed using dedicated chemical reactions in the collision/reaction cell; finally, the third quadrupole works to isolate the analyte and free it from any further interference.
Although ICP-MS methods may have been labeled as complicated in the past, its processes have been simplified greatly. Next-generation software integration can automate tasks and consolidate workflows to reduce user error and save time, freeing scientists to work on additional projects.
Putting ICP-MS to the Test
To test the real-world application of ICP-MS technology for the identification and quantification of REEs, Thermo Fisher Scientific’s team used its latest project — the Thermo Scientific™ iCAP™ TQe ICP-MS based on triple quadrupole technology, operated using a single measurement mode with oxygen as a reactive gas.
This technology was used to analyze two certified reference materials (CRMs) and eight water samples from various locations in and around Bremen, Germany (see Figure 1). 35 different analytes were tested, including major elements such as sodium, magnesium, potassium and iron, common analytes such as chromium, arsenic, and selenium, and 15 REEs. Promethium was not analyzed due to its radioactivity and scandium is not commonly analyzed as it is often used as an internal standard.
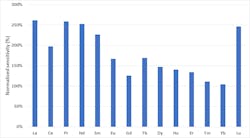
Due to the complex nature of the river water samples collected, predicted concentrations ranged from 0.01 µg·L-1 to 100,000 µg·L-1. Despite this wide concentration range and the complexity of the sample matrix, the ICP-MS system showed greater detection sensitivity compared to the kinetic energy detection commonly applied in single quadrupole ICP-MS. This is shown specifically for the group of lanthanoids (Figure 2).
In addition, the ICP-MS method reduced analysis time to less than three minutes per sample, proving that high sample throughputs are possible while achieving comparably higher sensitivity and similar levels of accuracy. The large linear dynamic ranges afforded by the ICP-MS system enabled precise determination at low and high concentrations without further sample dilution or concentration. This simplified workflows and helped to save valuable sample preparation time.
Despite the speed of analysis, the system was shown to be robust, supplying 10 hours of continuous acquisition of 200 samples. As final proof of the viability of the method, the analysis showed excellent agreement between the results and the CRMs, confirming an accurate and fast detection and quantification of REEs within the samples5.
Fast, Sensitive and Robust
The challenges of detecting trace amounts of REEs in complex samples with high spectral interference has meant that REE analysis is not often included as part of the standard suite of water testing in analytical laboratories. Indeed, REE analysis is often outsourced to environmental research studies, reducing the amount of valuable insight gained from standard water testing.
Next-generation ICP-MS technology, combined with automation software, now provides a way to tackle this complex problem. By incorporating REE analysis into methods already used to test for other key contaminants, water analysis laboratories can broaden their suite of services and provide much needed and valuable data. Scientists can now achieve fast, sensitive and robust results for trace REEs, even in complex and high-interference samples.
REE use is predicted to rise in coming years to meet the growing demand from accelerating markets such as electrical vehicle production6. As extraction and disposal rates increase, accurate detection and quantification will be ever more important to protect the environment and public health.
About the Author: Daniel Kutscher is Thermo Fisher Scientific's product specialist for ICP-MS technology. After graduating in Chemistry in 2007, Daniel obtained his PhD from the University of Oviedo in 2011. In the same year he joined Thermo Fisher Scientific in his role as Application Specialist for ICP-MS and joined the Marketing team in 2018. Over the years, Daniel was involved in finding solutions for the high throughput analysis of challenging sample types using ICP-MS.
References
Sabina Dołęgowska, Zdzisław M. Migaszewski. Anomalous concentrations of rare earth elements in the moss–soil system from south-central Poland. Environmental Pollution, Volume 178, 2013, pages 33-40, ISSN 0269-7491. https://doi.org/10.1016/j.envpol.2013.02.024
M. Olías, J.C. Cerón, I. Fernández, J. De la Rosa. Distribution of rare earth elements in an alluvial aquifer affected by acid mine drainage: the Guadiamar aquifer (SW Spain). Environmental Pollution, Volume 135, Issue 1, 2005, pages 53-64, ISSN 0269-7491. https://doi.org/10.1016/j.envpol.2004.10.014
Weifang Zhu, Suqin Xu, Pinpin Shao, Hui Zhang, Donseng Wu, Wenjia Yang, Jia Feng, Lei Feng. Investigation on liver function among population in high background of rare earth area in South China. Biological Trace Element Research volume 104, 2005, pages 1–7. https://link.springer.com/article/10.1385%2FBTER%3A104%3A1%3A001#Abs1 https://e360.yale.edu/features/china-wrestles-with-the-toxic-aftermath-of-rare-earth-mining https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/an-44479-icp-ms-rare-earth-elements-contaminants-an44479-en.pdf
Goodenough, K.M., Wall, F. & Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat Resour Res 27, 201–216 (2018). https://doi.org/10.1007/s11053-017-9336-5
About the Author
Daniel Kutscher
Daniel Kutscher is Thermo Fisher Scientific's product specialist for ICP-MS technology.
After graduating in Chemistry in 2007, Daniel obtained his PhD from the University of Oviedo in 2011. In the same year he joined Thermo Fisher Scientific in his role as Application Specialist for ICP-MS and joined the Marketing team in 2018. Over the years, Daniel was involved in finding solutions for the high throughput analysis of challenging sample types using ICP-MS.