Iron and Peroxide Chemistry
A common weapon for combating several wastewater treatment plant issues
By Brad Buecker, Jamie Belden and Inken Mello
Publicly owned treatment works (POTW) personnel must deal with a multitude of complex factors at their plants. Variable influent quality and quantity, process upsets, and plant discharge issues are of constant concern, coupled with the ever-present need to operate economically.
This article outlines a straightforward treatment method based on iron and peroxide chemistry that can potentially help plant personnel better control phosphorus discharge, reduce hydrogen sulfide odors, and mitigate the potential for struvite deposition, a common problem in wastewater plants that can clog pipes and other equipment.
Phosphorus: A Gorilla in the Room
Phosphorus is an essential element for life and as such, all forms of municipal waste contain it (typically as phosphate [PO4] compounds). Phosphate, however, can be problematic in wastewater treatment and industrial plants for several reasons.
In POTWs, phosphate will react with magnesium and ammonium ions to form the deposit struvite (NH4MgPO4•6H2O). Struvite deposition (see Fig. 1) may be particularly problematic in plants with anaerobic digesters, as will be discussed later.
On a much broader scale, phosphorus discharge from POTWs and other industrial facilities is increasingly being regulated and restricted due to serious problems from the formation of toxic algae blooms in receiving bodies of water.
Although algae blooms are commonly perceived to be most prominent in temperate climates such as Florida and the Gulf of Mexico, they can occur in colder environments as well (see Fig. 2). Thus, point source discharge restrictions for phosphorus are appearing in many areas of the country.
In yet another development, alternatives to fresh water are increasingly being selected for makeup to industrial plants. A leading alternative is secondary-treated effluent from POTWs. Unless removed upstream, phosphate, nitrogen compounds (ammonia and nitrite/nitrate), and organics provide substantial nutrients and food for microbiological growth within plant cooling and service water systems. The microorganisms can induce extreme fouling of heat exchangers, cooling tower fill, and other equipment (see Fig. 3).
It is critical to address these issues early in the design phase of any project and to institute measures to remove impurities upstream of the industrial facility. (As a side note, with regard to cleaning fouled cooling tower fill, peroxide chemistry similar to that highlighted in this article can be quite effective, provided the fill is not irreversibly fouled.1)
Phosphate Removal
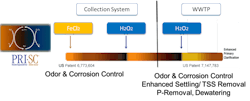
Techniques to remove phosphate prior to discharge of POTW effluent are of increasing necessity. A standard method is precipitation of phosphate with a metal salt, typically ferric chloride or ferric sulfate. The ferric compounds are also good at oxidizing and removing sulfide (H2S), thus contributing significantly to odor control. It is within these processes that supplemental use of hydrogen peroxide (H2O2) assists with iron recovery and reuse (an economic benefit), additional odor control, and permanent sequestration of phosphate, which in turn reduces the potential for struvite formation (see Fig. 4).
Full-Scale Applications
At the Water Reclaim Facility (WRF) in Wichita, Kans., several primary treatment methods, including aerobic processes, are utilized to initially condition the incoming wastewater (see Fig. 5). Clear water discharge from the final clarifiers receives ultraviolet (UV) disinfection and reaeration prior to discharge.
A key component of the overall process is feed of ferric iron at various points in the system, where usage often averages around 1,000 lbs/day. Ferric salts, typically chloride or sulfate, may serve several functions at a POTW:
• Coagulating agent for the clarifiers
• Odor control by binding sulfide from H2S
• Phosphate precipitation and transfer of phosphate from liquid to solid phase
It is in respect to the latter two items that the PRI-TECH® methodology has proven to be a valuable modification. The beneficial microorganisms in the anaerobic digesters induce the re-release of phosphorus, primarily as orthophosphate (PO4). Thus, digester effluent contains magnesium, ammonium, and phosphate ions, which can lead to the formation of struvite deposits in post-digester pumps, piping, tanks, and filter press dewatering piping.
Wichita WRF personnel conducted successful trials of ferric chloride injection to the digester effluent/filter press influent to reduce the dissolved phosphate concentration. The tests indicated a reduction in dissolved phosphate concentration of the BFP filtrate from an average of 85 mg/L to less than 20 mg/L. But it is here where peroxide injection shows an added advantage. The 1,000 lbs/day on average of ferric iron fed to the system equates to around a 150 to 200 mg/L residual of spent iron in the digester solids. Injection of peroxide reactivates the spent iron in the digester sludge by oxidizing the sulfide from the ferrous sulfide (FeS) complex, which correspondingly lowers the more expensive ferric chloride (FeCl3) feed needed at this location.
Other advantages were also noted after start-up of this technology. The H2S vapor concentration was reduced from 16 ppm to essentially non-detectable in the biosolids feed pump room, and final cake solids increased in concentration by up to 6 percent. These solids are stored on-site, and then are applied to farmland as conditions permit. Long-term studies are underway to determine the effects on crop growth from the increase in bound phosphate due to the peroxide process enhancement.
A similar program has been established at another plant in the Southwest. Primary-treated wastewater is first conditioned for suspended solids removal in a set of upstream clarifiers with ferric sulfate [Fe2(SO4)3] as the coagulant. Besides serving as a coagulant, the ferric salt also reacts with phosphate to transfer a substantial portion of the phosphate to the clarifier blowdown sludge. The clarifier effluent is routed to aeration basins for further aerobic treatment while the sludge is routed through gravity thickeners and then is transferred to three anaerobic digesters.
The thickener inlet stream is treated with both ferric sulfate and peroxide. This stage is an initial odor control step in the process. As with the previous example, ferric chloride is fed to the digester effluent/belt filter influent to precipitate phosphate and reduce odors. However, a PRI-TECH® system has been installed to treat the effluent from two of the digesters. These two digesters handle approximately 60 percent of the waste flow.
The initial results of the peroxide feed indicate a 50 percent reduction in ferric chloride feed, with 60 percent reduction anticipated as the system is fine tuned. The projected annual chemical cost savings due to the initial peroxide feed modification will be greater than $100,000. Furthermore, based on the very positive results so far, plans are in progress to convert the third digester effluent stream to this process. After fine tuning, annual chemical cost savings are projected to exceed $200,000.
Conclusion
The real-world examples above are another illustration of the evolution of improved technologies for water treatment applications. They also represent examples of “green” chemistry, as hydrogen peroxide leaves no residual elements or compounds to handle, unlike chlorine or other halogen-based oxidizing chemicals. The breakdown products of H2O2 are oxygen and water, which is as benign as it gets. Note, however, that all systems are different and have their own peculiarities. Each application requires careful evaluation of process conditions and consultation with feed system experts to ensure correct installation and operation. WW
References
1. Buecker, B. “Cleaning Cooling Tower Fill,” Industrial WaterWorld, May/June 2019.Circle No. 239 on Reader Service Card
About the Authors: Brad Buecker has 35 years of experience in or affiliated with the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering. He is currently senior technical publicist with ChemTreat.
Jamie Belden is the sewage treatment operations supervisor for the City of Wichita, Kans.
Inken Mello is the business development manager for USP Technologies specializing in chemical solutions for odor and corrosion control, as well as phosphorus removal, struvite control, and dewatering enhancements for wastewater treatment plants.
About the Author
Brad Buecker
Brad Buecker has 35 years of experience in or affiliated with the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering. He is currently senior technical publicist with ChemTreat.
Jamie Belden
Jamie Belden is the sewage treatment operations supervisor for the City of Wichita, Kans.
Inken Mello
Inken Mello is the business development manager for USP Technologies specializing in chemical solutions for odor and corrosion control, as well as phosphorus removal, struvite control, and dewatering enhancements for wastewater treatment plants.